CH 362 Virtual lecture January 20 2020 Esterification





















- Slides: 22

CH 362 Virtual lecture January 20, 2020 Esterification of Acids

Recap of Project 1 OREGON STATE UNIVERSITY 2

Recap of Project 1 OREGON STATE UNIVERSITY 3

Esterification: A nucleophilic substitution of an OH group OREGON STATE UNIVERSITY 4

Esterification: A nucleophilic substitution of an OH group Basic conditions: no good Deprotonation of acid makes it too poor an electrophile OREGON STATE UNIVERSITY 5

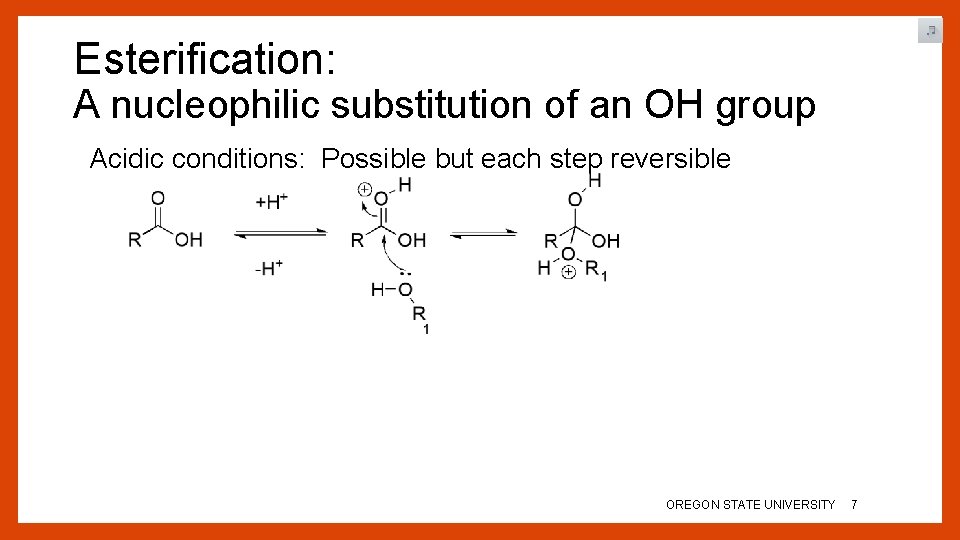
Esterification: A nucleophilic substitution of an OH group Acidic conditions: Possible but each step reversible OREGON STATE UNIVERSITY 6

Esterification: A nucleophilic substitution of an OH group Acidic conditions: Possible but each step reversible OREGON STATE UNIVERSITY 7

Esterification: A nucleophilic substitution of an OH group Acidic conditions: Possible but each step reversible OREGON STATE UNIVERSITY 8

Esterification: A nucleophilic substitution of an OH group Acidic conditions: Possible but each step reversible If each step is reversible the overall process is reversible OREGON STATE UNIVERSITY 9

Esterification: A nucleophilic substitution of an OH group Acidic conditions: Possible but each step reversible Possible undesired side Reaction if ester exposed to Water & acid! If each step is reversible the overall process is reversible OREGON STATE UNIVERSITY 10

Esterification: A nucleophilic substitution of an OH group Activate OH (turn it into an irreversible leaving group) OREGON STATE UNIVERSITY 11

Esterification: A nucleophilic substitution of an OH group Activate OH (turn it into an irreversible leaving group) OREGON STATE UNIVERSITY 12

Esterification: A nucleophilic substitution of an OH group Activate OH (turn it into an irreversible leaving group) OREGON STATE UNIVERSITY 13

Esterification: A nucleophilic substitution of an OH group Activate OH (turn it into an irreversible leaving group) OREGON STATE UNIVERSITY 14

Esterification: A nucleophilic substitution of an OH group Activate OH (turn it into an irreversible leaving group) OREGON STATE UNIVERSITY 15

Esterification: A nucleophilic substitution of an OH group Activate OH (turn it into an irreversible leaving group) Each step is irreversible, driven by formation of SO 2 and HCl. DMF is regenerated and so is a catalyst. See Tetrahedron, 2005, 61, 10827 for details OREGON STATE UNIVERSITY 16

Reaction of the Acid Chloride with an Alcohol Is Also Irreversible OREGON STATE UNIVERSITY 17

Reaction of the Acid Chloride with an Alcohol Is Also Irreversible OREGON STATE UNIVERSITY 18

Reaction of the Acid Chloride with an Alcohol Is Also Irreversible OREGON STATE UNIVERSITY 19

Reaction of the Acid Chloride with an Alcohol Is Also Irreversible OREGON STATE UNIVERSITY 20

Reaction of the Acid Chloride with an Alcohol Is Also Irreversible OREGON STATE UNIVERSITY 21

Workup: Remove HCl, water, vacuum distill. HCl will catalyze hydrolysis. Wash with Na. HCO 3 until aqueous layer basic. Water will slowly hydrolyze the ester, even with no catalysis. Wash with saturated Na. Cl, dry with Mg. SO 4 Vacuum distillation will ensure purity. The P ester is difficult to separate from n-butanol. Limit the amount of butanol used; be careful in cutting fractions. (See CH 361 material on fractional Distillation; Mohrig Ch. 12 OREGON STATE UNIVERSITY 22