Ch 3 The Canonical Ensemble Microcanonical ensemble describes
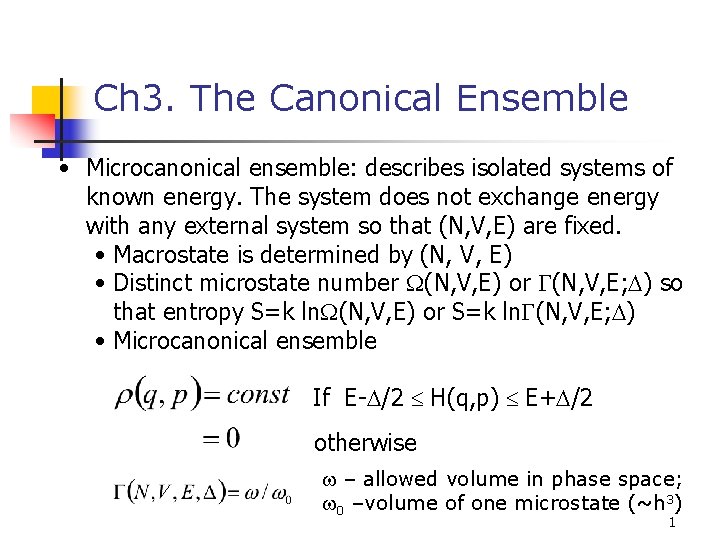
Ch 3. The Canonical Ensemble • Microcanonical ensemble: describes isolated systems of known energy. The system does not exchange energy with any external system so that (N, V, E) are fixed. • Macrostate is determined by (N, V, E) • Distinct microstate number W(N, V, E) or G(N, V, E; D) so that entropy S=k ln. W(N, V, E) or S=k ln. G(N, V, E; D) • Microcanonical ensemble If E-D/2 £ H(q, p) £ E+D/2 otherwise w – allowed volume in phase space; w 0 –volume of one microstate (~h 3) 1
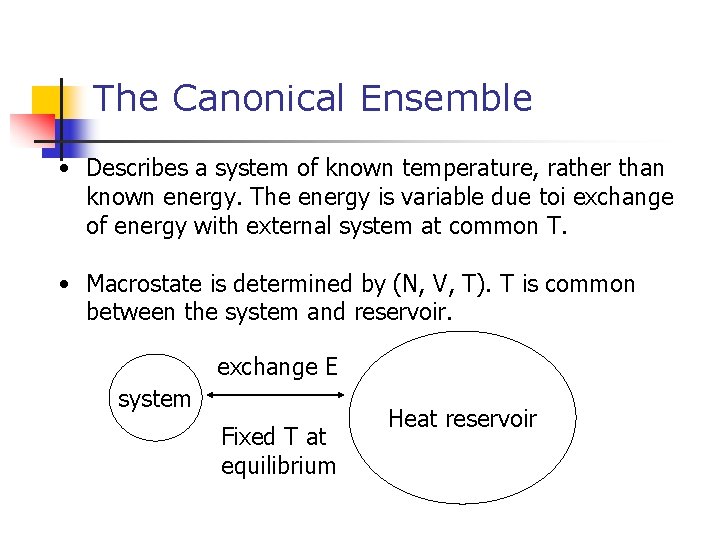
The Canonical Ensemble • Describes a system of known temperature, rather than known energy. The energy is variable due toi exchange of energy with external system at common T. • Macrostate is determined by (N, V, T). T is common between the system and reservoir. exchange E system Fixed T at equilibrium Heat reservoir
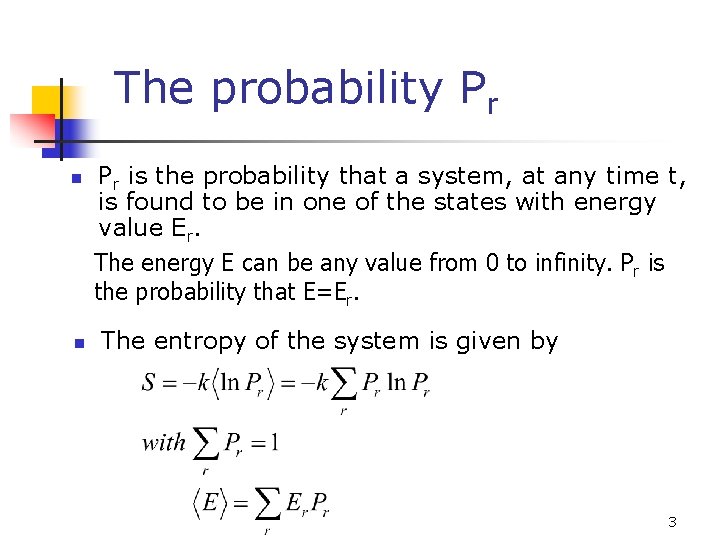
The probability Pr n n Pr is the probability that a system, at any time t, is found to be in one of the states with energy value Er. The energy E can be any value from 0 to infinity. Pr is the probability that E=Er. The entropy of the system is given by 3
Pr in microcanonical ensemble Each mocrostate is equally accessible 4
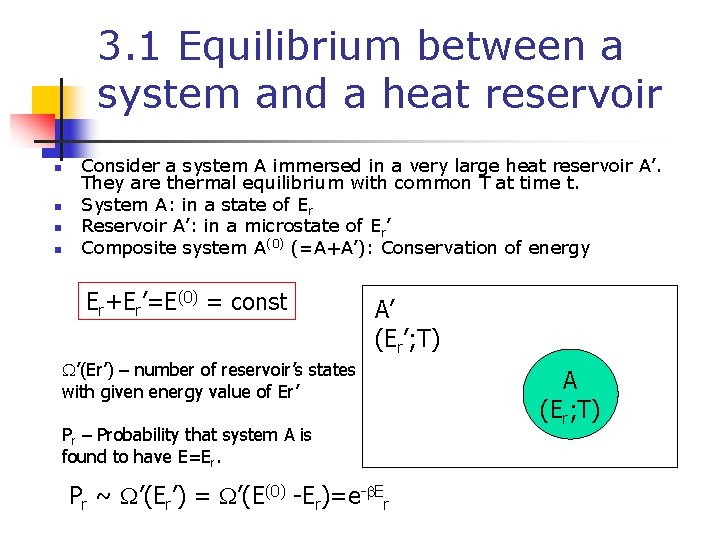
3. 1 Equilibrium between a system and a heat reservoir n n Consider a system A immersed in a very large heat reservoir A’. They are thermal equilibrium with common T at time t. System A: in a state of Er Reservoir A’: in a microstate of Er’ Composite system A(0) (=A+A’): Conservation of energy Er+Er’=E(0) = const A’ (Er’; T) W’(Er’) – number of reservoir’s states with given energy value of Er’ Pr – Probability that system A is found to have E=Er. Pr ~ W’(Er’) = W’(E(0) -Er)=e-b. Er A (Er; T)
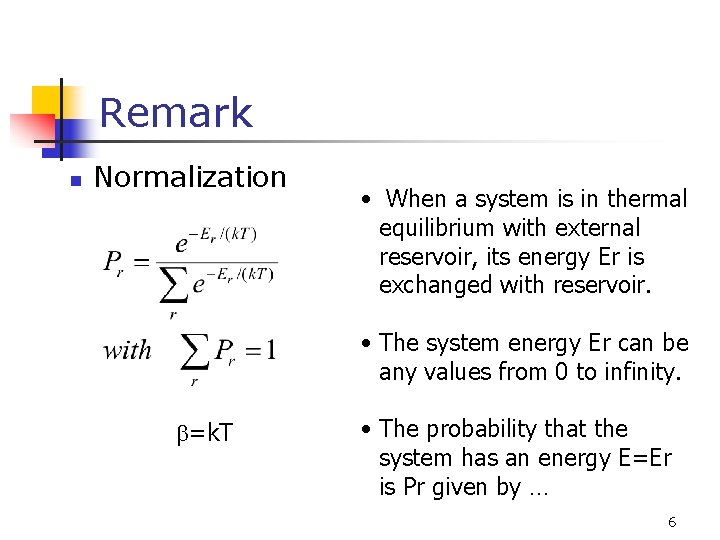
Remark n Normalization • When a system is in thermal equilibrium with external reservoir, its energy Er is exchanged with reservoir. • The system energy Er can be any values from 0 to infinity. b=k. T • The probability that the system has an energy E=Er is Pr given by … 6
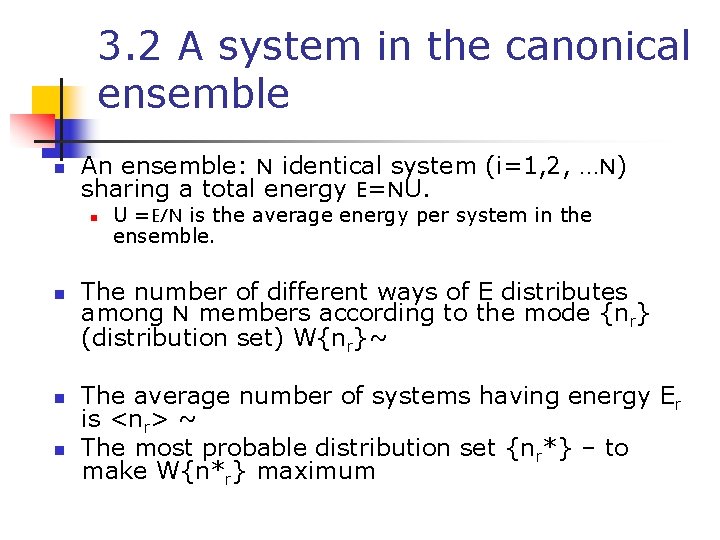
3. 2 A system in the canonical ensemble n An ensemble: N identical system (i=1, 2, …N) sharing a total energy E=NU. n n U =E/N is the average energy per system in the ensemble. The number of different ways of E distributes among N members according to the mode {nr} (distribution set) W{nr}~ The average number of systems having energy Er is <nr> ~ The most probable distribution set {nr*} – to make W{n*r} maximum
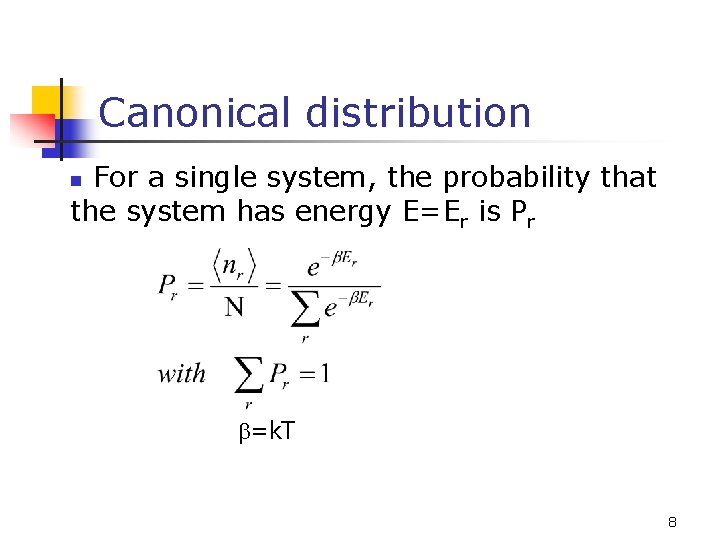
Canonical distribution For a single system, the probability that the system has energy E=Er is Pr n b=k. T 8

3. 3 Physical significance of the various statistical quantities in the canonical ensemble n The canonical distribution- the probability that the system has energy E=Er b=k. T n Partition function of the system with (N, V, T) n The average energy of the system with (N, V, T)
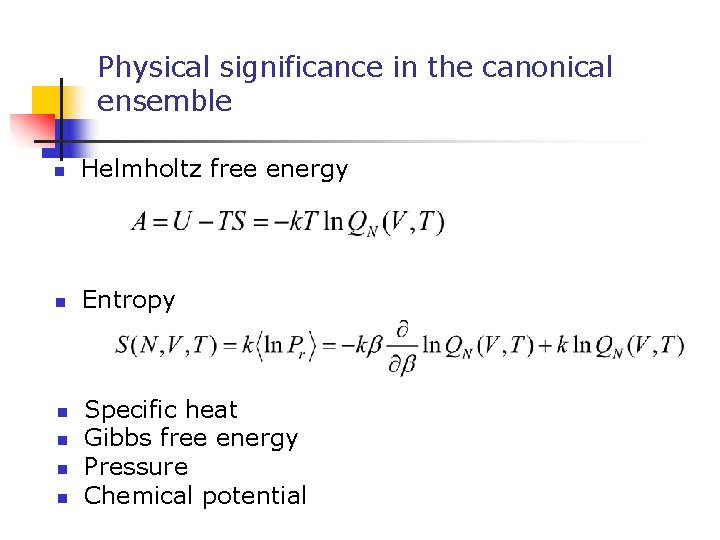
Physical significance in the canonical ensemble n Helmholtz free energy n Entropy n n Specific heat Gibbs free energy Pressure Chemical potential
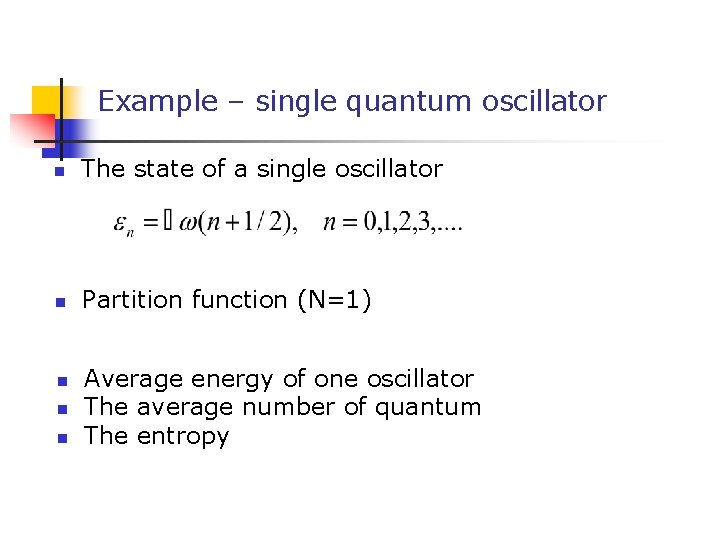
Example – single quantum oscillator n The state of a single oscillator n Partition function (N=1) n n n Average energy of one oscillator The average number of quantum The entropy
- Slides: 11