Ch 3 Textures Some Useful Definitions l l





















- Slides: 39

Ch. 3: Textures Some Useful Definitions l l The Surface Energy is the excess energy at the surface of a material (unbalanced ionic charges, etc. ) compared to the interior. Surface energy is measured in the laboratory as the energy (work = force x distance) consumed while cutting a new surface in the plane of interest.

Some Useful Definitions (cont. ) l l Instability: the spontaneous separation of small clusters of compatible ions due to high surface charge. This inhibits nucleation. Nucleation: forming a critical-sized embryonic crystal, a crystal that is large enough to have sufficient balanced interior volume, so that it is stable and won’t fly apart due to like electrostatic charges. Undercooling: cooling of a melt below the true crystallization temperature of a mineral. Twin: an intergrowth of two or more orientations of the same mineral that share common atoms, typically on a plane.

Nucleation l l Requires either undercooling or A seed crystal of same or similar structure mineral l Observations l Simple structures nucleate more easily Oxides easier > Olivines > Pyrox. > Plag. > K-spars l So oxides small & numerous, K-spars few l

Diffusion l l For growth to proceed, constituents must diffuse through the melt, cross the depleted zone, and reach the crystal surface. Crystal formation produces heat “Latent heat of crystallization” This heat must diffuse away, or the temperature (KE) may become too high for crystallization to proceed.

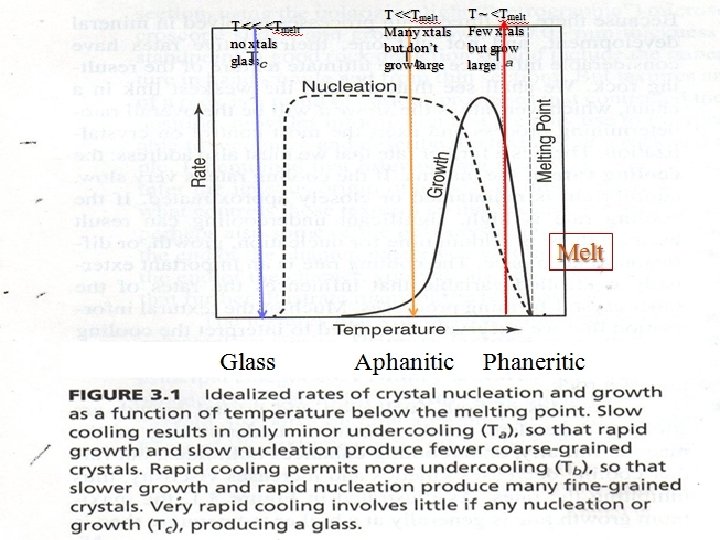
T << <Tmelt no xtals glass T <<Tmelt Many xtals but don’t grow large T ~ <Tmelt Few xtals but grow large Melt Glass Aphanitic Phaneritic

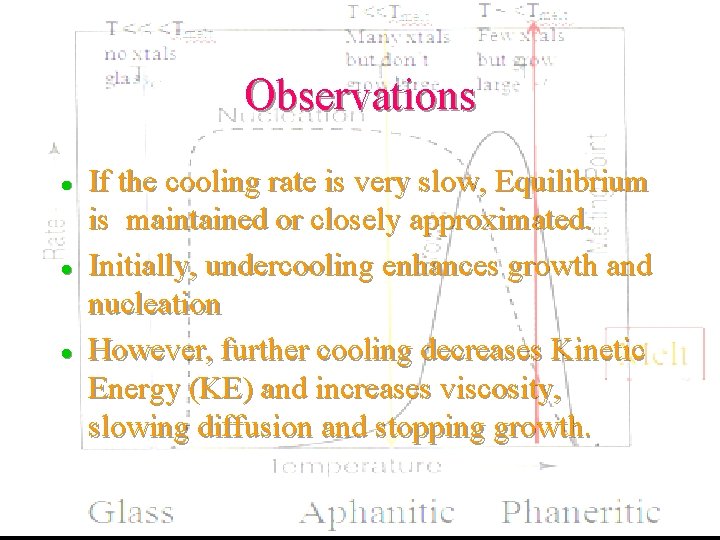
Observations l l l If the cooling rate is very slow, Equilibrium is maintained or closely approximated. Initially, undercooling enhances growth and nucleation However, further cooling decreases Kinetic Energy (KE) and increases viscosity, slowing diffusion and stopping growth.

Porphyritic l l l Rock with distinct difference in xtal sizes. Igneous: slow cooling in magma chamber, fast cooling near/at surface. Phenocrysts In glassy matrix/groundmass vitrophyric If phenocrysts contain numerous inclusions poikilitic.

Large Crystal Growth l l l Largest xtals have The most plentiful components in adjacent melt The fastest diffusing components Faster at higher temps MAFIC F Faster in material of low viscosity MAFIC F Slower in highly polymerized viscous melts FELSIC F Diffusion fastest in fluid > glass > solid F

Role of Water and Pegmatites l l H 2 O dramatically reduces the polymerization of magma. Melt is less viscous. Large xtal size in Pegmatites may be attributed more to high diffusion of components through the H 2 O-rich melt, rather than just slow cooling.

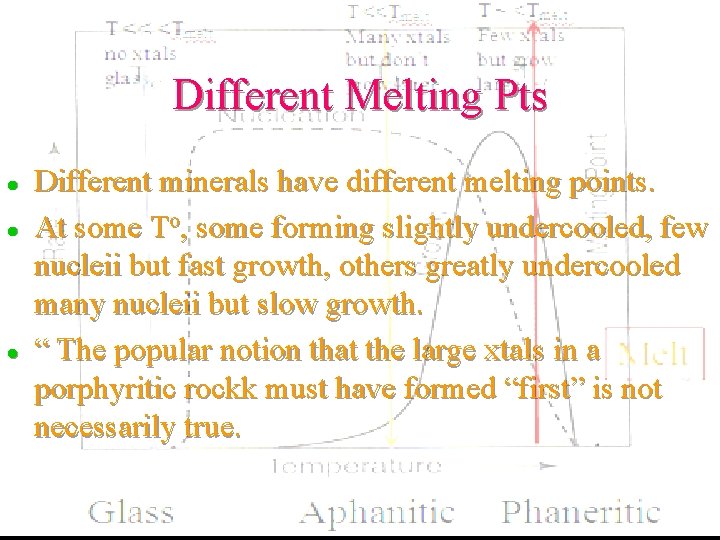
Different Melting Pts l l l Different minerals have different melting points. At some To, some forming slightly undercooled, few nucleii but fast growth, others greatly undercooled many nucleii but slow growth. “ The popular notion that the large xtals in a porphyritic rockk must have formed “first” is not necessarily true.

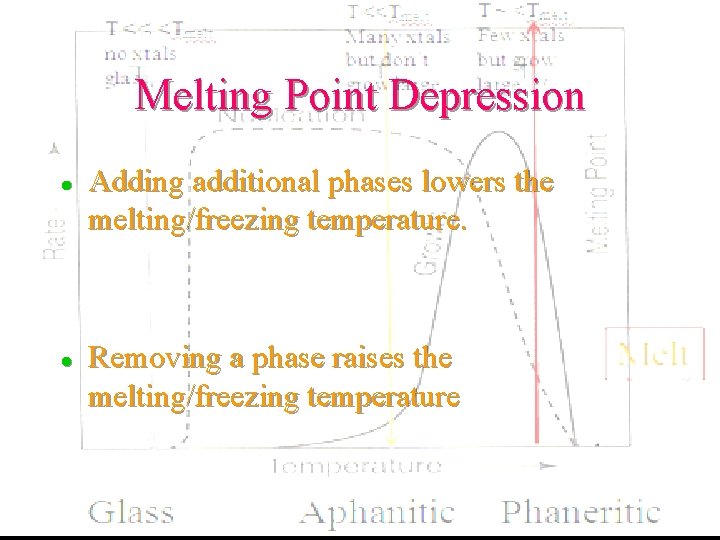
Melting Point Depression l l Adding additional phases lowers the melting/freezing temperature. Removing a phase raises the melting/freezing temperature

Dewatering l Sudden loss of the water-rich fluid phase will quickly raise the melting point, making the forming minerals significantly more undercooled at the current Temperature. This can move mineral species to form many new nuclei, with low growth, producing many aphanitic crystals. This can produce a porphyritic texture in plutonic rocks if phenocrysts were already present.

Crystal Growth l l l Addition of more ions onto existing face Observations: Bravais: planes with a high density of lattice points form a more prominent face Fast growing faces have smaller interplanar distances Faces with low surface energy become more prevalent

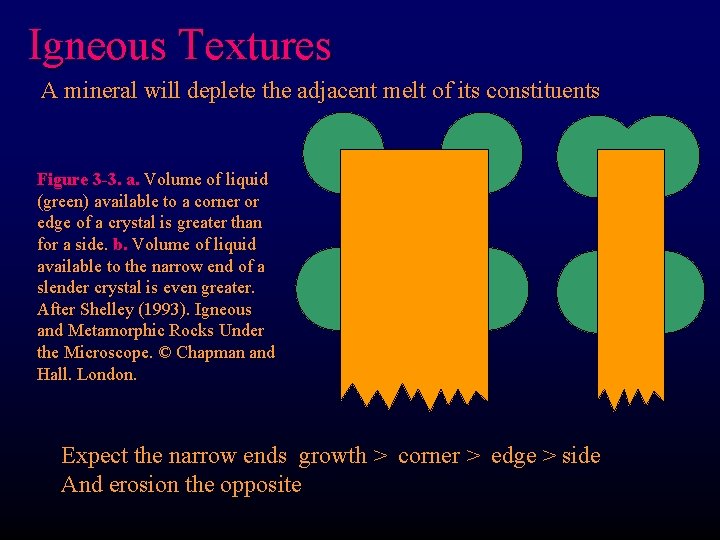
Igneous Textures A mineral will deplete the adjacent melt of its constituents Figure 3 -3. a. Volume of liquid (green) available to a corner or edge of a crystal is greater than for a side. b. Volume of liquid available to the narrow end of a slender crystal is even greater. After Shelley (1993). Igneous and Metamorphic Rocks Under the Microscope. © Chapman and Hall. London. a Expect the narrow ends growth > corner > edge > side And erosion the opposite b

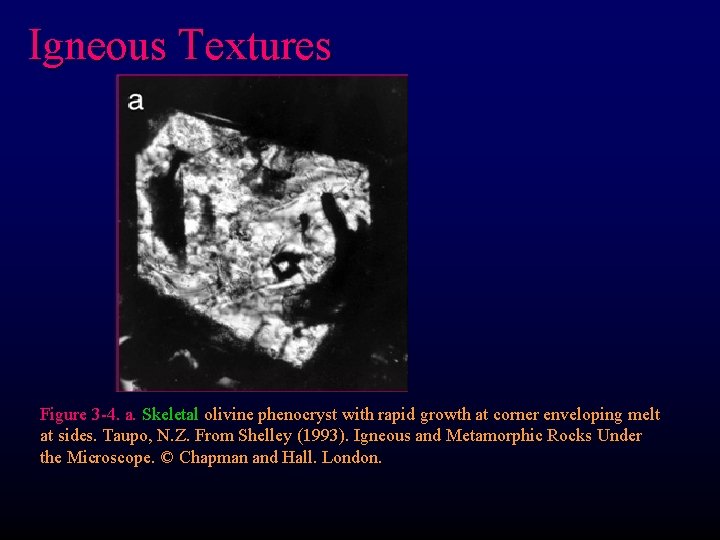
Igneous Textures Figure 3 -4. a. Skeletal olivine phenocryst with rapid growth at corner enveloping melt at sides. Taupo, N. Z. From Shelley (1993). Igneous and Metamorphic Rocks Under the Microscope. © Chapman and Hall. London.

Epitaxis l l Preferred nucleation of one mineral on another pre-existing mineral. Ex. 1: Sillimanite on mica rather than on its polymorph Kyanite in equilibrium Si-Al-O structures more similar in the micas. Ex. 2: Plagioclase overgrows K-spar Orthoclase rather than nucleate on its own.

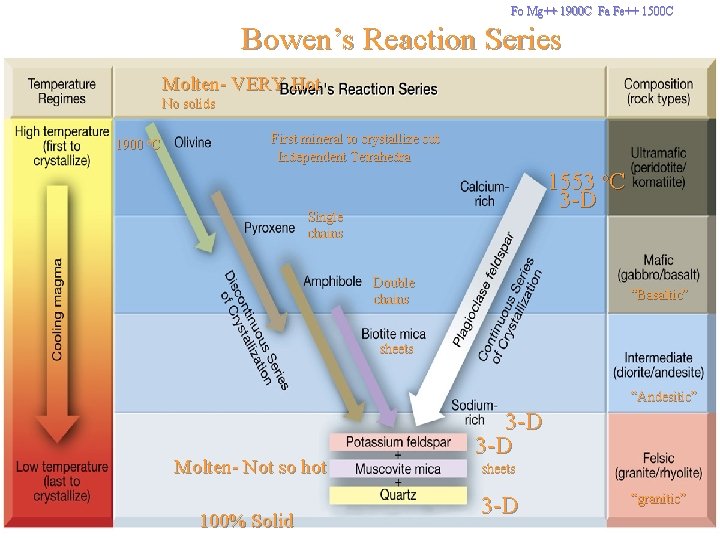
Fo Mg++ 1900 C Fa Fe++ 1500 C Bowen’s Reaction Series Molten- VERY Hot No solids 1900 o. C First mineral to crystallize out Independent Tetrahedra 1553 o. C 3 -D Single chains Double chains “Basaltic” sheets “Andesitic” Molten- Not so hot 100% Solid 3 -D sheets 3 -D “granitic”

Compositional Zoning Figure 3 -5. a. Compositionally zoned hornblende phenocryst with pronounced color variation visible in plane-polarized light. Field width 1 mm. b. Zoned plagioclase twinned on the Carlsbad law. (twins 180 on b) Andesite, Crater Lake, OR. Field width 0. 3 mm. © John Winter and Prentice Hall. Take home lesson: if you leave a crystal exposed to the melt, the melt will react with it, sometimes scavenging parts. Olivine examples seen in Basalts If a new mineral is stable at new conditions, the old mineral will be covered with the new. This example of Plagioclase in a cooling melt.

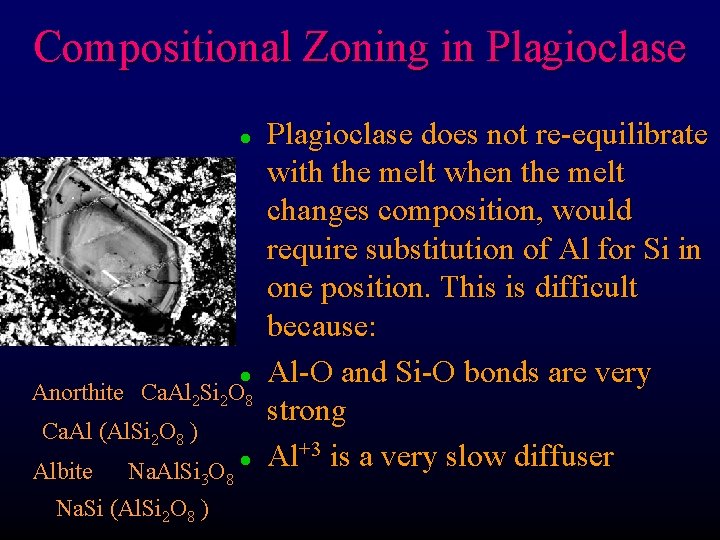
Compositional Zoning in Plagioclase l Anorthite l Ca. Al 2 Si 2 O 8 Ca. Al (Al. Si 2 O 8 ) Albite Na. Al. Si 3 O 8 Na. Si (Al. Si 2 O 8 ) l Plagioclase does not re-equilibrate with the melt when the melt changes composition, would require substitution of Al for Si in one position. This is difficult because: Al-O and Si-O bonds are very strong Al+3 is a very slow diffuser

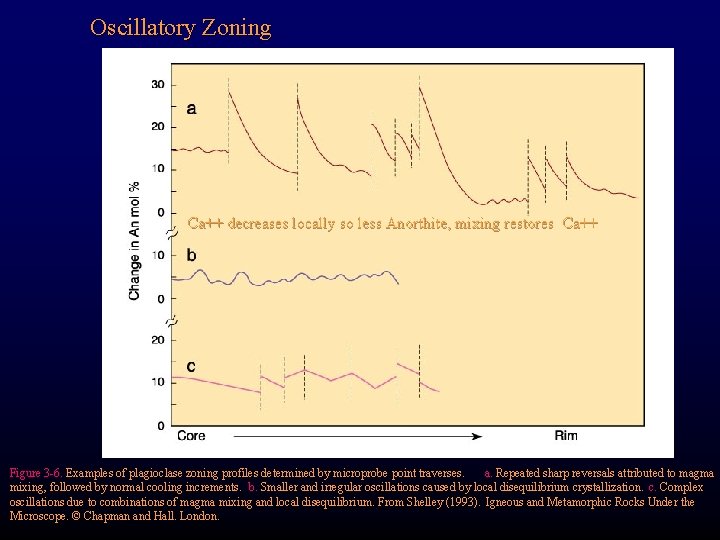
Oscillatory Zoning Ca++ decreases locally so less Anorthite, mixing restores Ca++ Figure 3 -6. Examples of plagioclase zoning profiles determined by microprobe point traverses. a. Repeated sharp reversals attributed to magma mixing, followed by normal cooling increments. b. Smaller and irregular oscillations caused by local disequilibrium crystallization. c. Complex oscillations due to combinations of magma mixing and local disequilibrium. From Shelley (1993). Igneous and Metamorphic Rocks Under the Microscope. © Chapman and Hall. London.

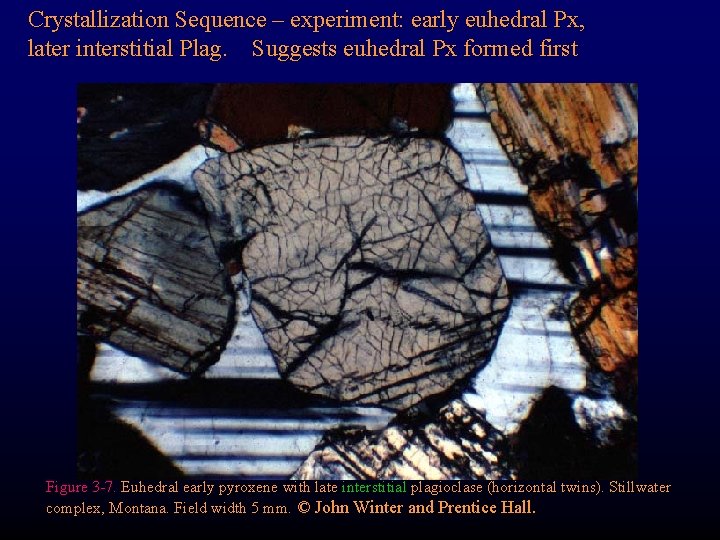
Crystallization Sequence – experiment: early euhedral Px, later interstitial Plag. Suggests euhedral Px formed first Figure 3 -7. Euhedral early pyroxene with late interstitial plagioclase (horizontal twins). Stillwater complex, Montana. Field width 5 mm. © John Winter and Prentice Hall.

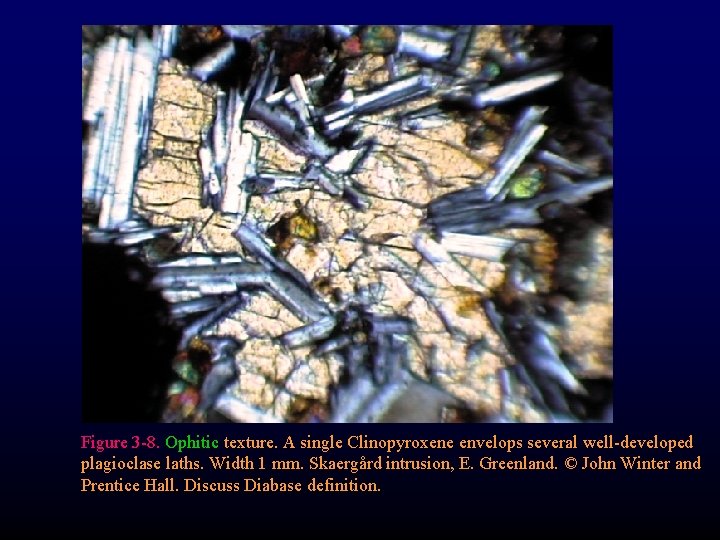
Figure 3 -8. Ophitic texture. A single Clinopyroxene envelops several well-developed plagioclase laths. Width 1 mm. Skaergård intrusion, E. Greenland. © John Winter and Prentice Hall. Discuss Diabase definition.

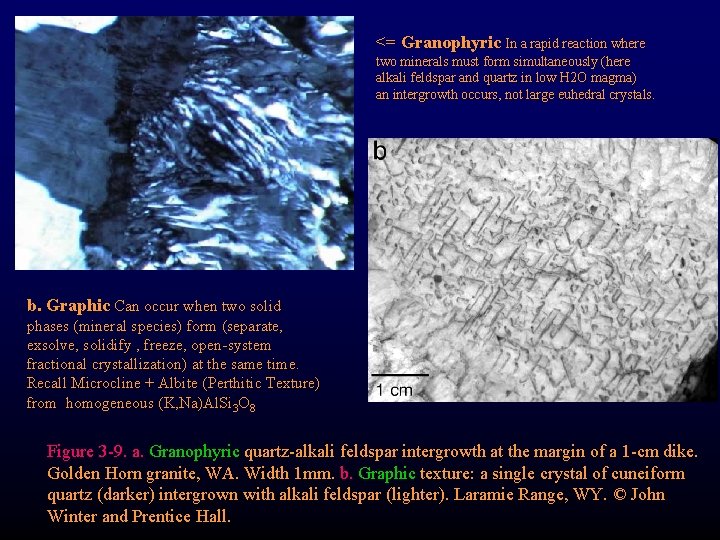
<= Granophyric In a rapid reaction where two minerals must form simultaneously (here alkali feldspar and quartz in low H 2 O magma) an intergrowth occurs, not large euhedral crystals. b. Graphic Can occur when two solid phases (mineral species) form (separate, exsolve, solidify , freeze, open-system fractional crystallization) at the same time. Recall Microcline + Albite (Perthitic Texture) from homogeneous (K, Na)Al. Si 3 O 8 Figure 3 -9. a. Granophyric quartz-alkali feldspar intergrowth at the margin of a 1 -cm dike. Golden Horn granite, WA. Width 1 mm. b. Graphic texture: a single crystal of cuneiform quartz (darker) intergrown with alkali feldspar (lighter). Laramie Range, WY. © John Winter and Prentice Hall.

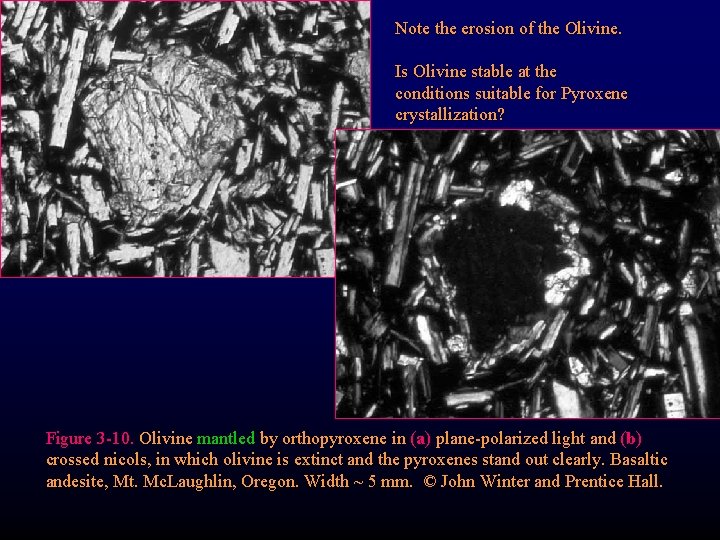
Note the erosion of the Olivine. Is Olivine stable at the conditions suitable for Pyroxene crystallization? Figure 3 -10. Olivine mantled by orthopyroxene in (a) plane-polarized light and (b) crossed nicols, in which olivine is extinct and the pyroxenes stand out clearly. Basaltic andesite, Mt. Mc. Laughlin, Oregon. Width ~ 5 mm. © John Winter and Prentice Hall.

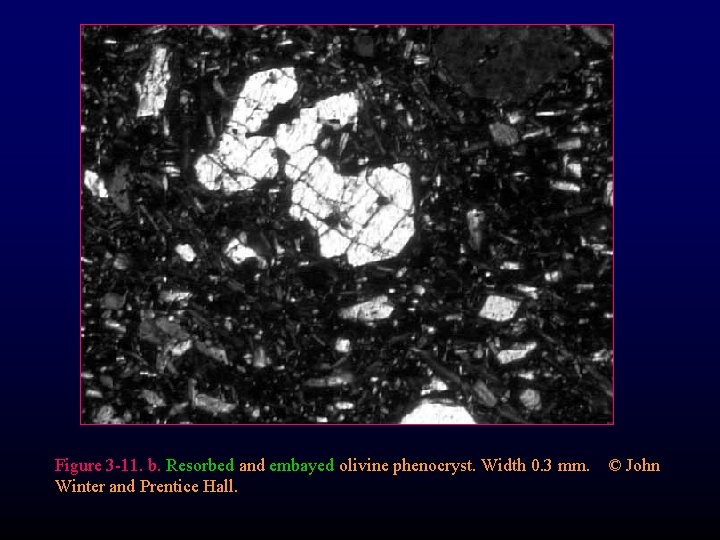
Figure 3 -11. b. Resorbed and embayed olivine phenocryst. Width 0. 3 mm. © John Winter and Prentice Hall.

When a hydrous magma reaches the surface, decompression releases volatiles, and hydrous minerals such as hornblende and biotite may develop rims of fine iron oxides and pyroxenes. Figure 3 -11. c. Hornblende phenocryst dehydrating to Fe-oxides plus pyroxene due to pressure release upon eruption. Andesite, Crater Lake, OR. Width 1 mm. © John Winter and Prentice Hall.

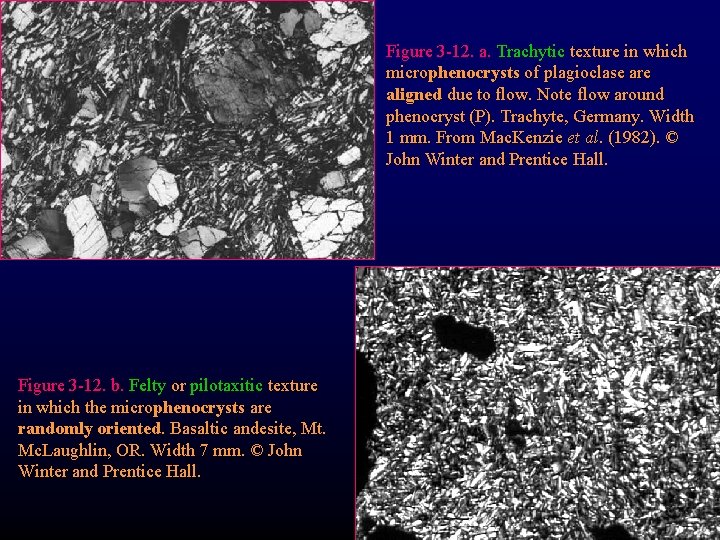
Figure 3 -12. a. Trachytic texture in which microphenocrysts of plagioclase are aligned due to flow. Note flow around phenocryst (P). Trachyte, Germany. Width 1 mm. From Mac. Kenzie et al. (1982). © John Winter and Prentice Hall. Figure 3 -12. b. Felty or pilotaxitic texture in which the microphenocrysts are randomly oriented. Basaltic andesite, Mt. Mc. Laughlin, OR. Width 7 mm. © John Winter and Prentice Hall.

Figure 3 -13. Flow banding in andesite. Mt. Rainier, WA. © John Winter and Prentice Hall. Caused by mingling of two magmatic fluids Figure 3 -15. Intergranular texture in basalt. Columbia River Basalt Group, Washington. Width 1 mm. © John Winter and Prentice Hall. Intergranular Plagioclase and Pyroxene crystals are similar in size.

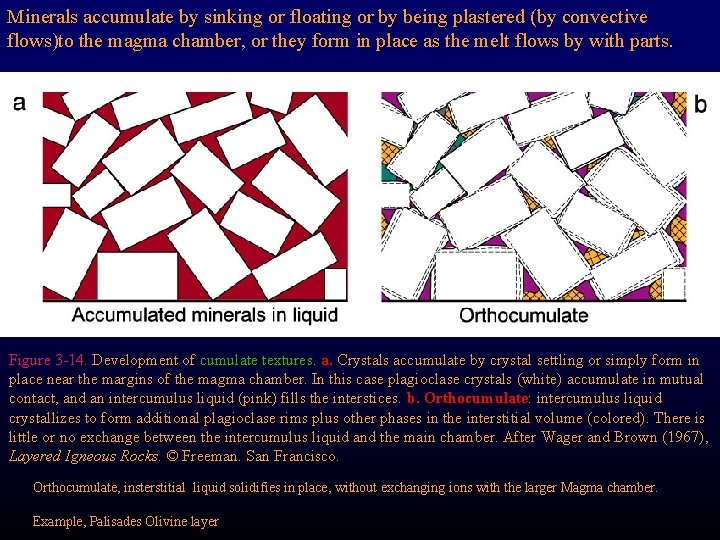
Minerals accumulate by sinking or floating or by being plastered (by convective flows)to the magma chamber, or they form in place as the melt flows by with parts. Figure 3 -14. Development of cumulate textures. a. Crystals accumulate by crystal settling or simply form in place near the margins of the magma chamber. In this case plagioclase crystals (white) accumulate in mutual contact, and an intercumulus liquid (pink) fills the interstices. b. Orthocumulate: intercumulus liquid crystallizes to form additional plagioclase rims plus other phases in the interstitial volume (colored). There is little or no exchange between the intercumulus liquid and the main chamber. After Wager and Brown (1967), Layered Igneous Rocks. © Freeman. San Francisco. Orthocumulate, insterstitial liquid solidifies in place, without exchanging ions with the larger Magma chamber. Example, Palisades Olivine layer

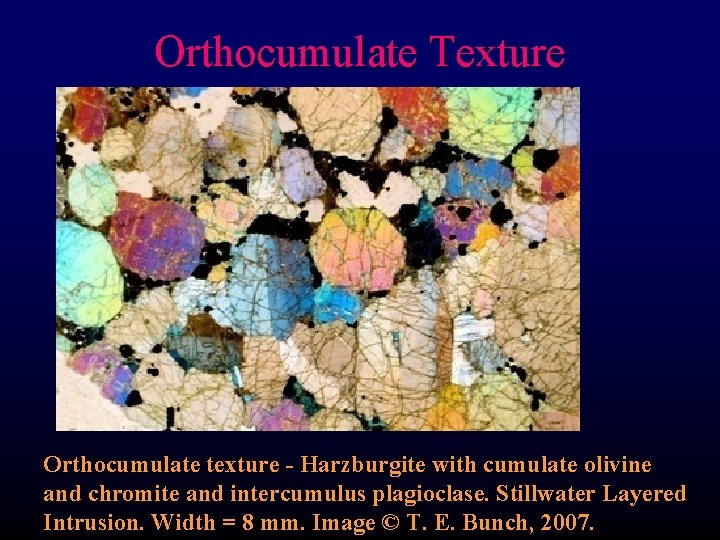
Orthocumulate Texture Orthocumulate texture - Harzburgite with cumulate olivine and chromite and intercumulus plagioclase. Stillwater Layered Intrusion. Width = 8 mm. Image © T. E. Bunch, 2007.

Figure 3 -14. Development of cumulate textures. c. Adcumulates: open-system exchange between the intercumulus liquid and the main chamber (plus compaction of the cumulate pile) allows components that would otherwise create additional intercumulus minerals to escape, and plagioclase fills most of the available space. d. Heteradcumulate: intercumulus liquid crystallizes to additional plagioclase rims, plus other large minerals (hatched and shaded) that nucleate poorly and poikilitically envelop the plagioclases. . After Wager and Brown (1967), Layered Igneous Rocks. © Freeman. San Francisco. Poikilitic: a mineral contains randomly oriented crystals of another mineral.

Poikilitic Texture Low int. color, 2 cleavages ~ 90 o, so Fledspar. Note no Albite twins, so K-spar. No Tartan twins so probably Sanadine or Orthoclase “In this photomicrograph, euhedral to subhedral biotite and plagioclase crystals are surrounded by optically-continuous, graycolored K-feldspar. ”

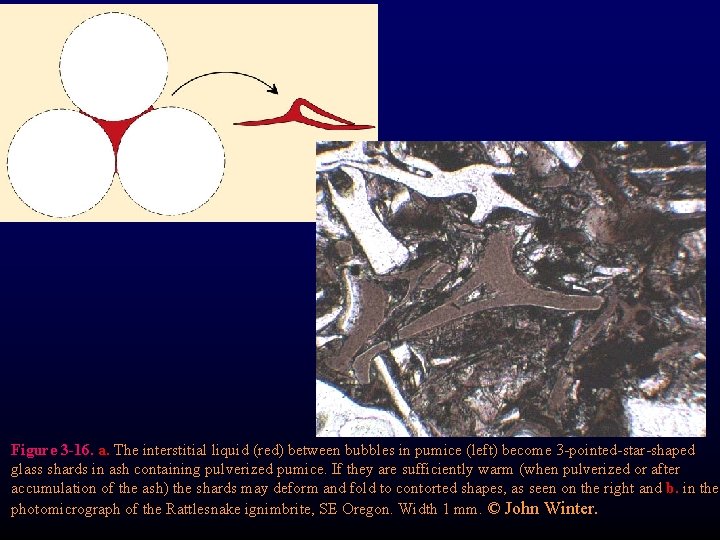
Figure 3 -16. a. The interstitial liquid (red) between bubbles in pumice (left) become 3 -pointed-star-shaped glass shards in ash containing pulverized pumice. If they are sufficiently warm (when pulverized or after accumulation of the ash) the shards may deform and fold to contorted shapes, as seen on the right and b. in the photomicrograph of the Rattlesnake ignimbrite, SE Oregon. Width 1 mm. © John Winter.

Figure 3 -18. a. Carlsbad twin in orthoclase. Wispy perthitic exsolution is also evident. Granite, St. Cloud MN. Field widths ~1 mm. © John Winter and Prentice Hall. Figure 3 -18. b. Very straight multiple albite twins in plagioclase, set in felsitic groundmass. Rhyolite, Chaffee, CO. Field widths ~1 mm. © John Winter and Prentice Hall.

Figure 3 -18. (c-d) Tartan twins in microcline. Field widths ~1 mm. © John Winter and Prentice Hall.

Figure 3 -19. Polysynthetic deformation twins in plagioclase. Note how they concentrate in areas of deformation, such as at the maximum curvature of the bent cleavages, and taper away toward undeformed areas. Gabbro, Wollaston, Ontario. Width 1 mm. © John Winter and Prentice Hall.

Figure 3 -20. a. Pyroxene largely replaced by hornblende. Some pyroxene remains as light areas (Pyx) in the hornblende core. Width 1 mm. b. Chlorite (green) replaces biotite (dark brown) at the rim and along cleavages. Tonalite. San Diego, CA. Width 0. 3 mm. © John Winter and Prentice Hall. Pyx Hbl If water infiltrates at moderate temperatures, pyroxenes are altered to amphiboles, and Biotite to Chlorite Chl Bt

Sericites and Sericitic Texture l The feldspars in this Alaskite (a low mafic granite) from the Boulder Batholith have been largely replaced by fine-grained Muscovite. This texture is called sericitic.

Myrmekitic As Plagioclase replaces a K-spar , silica Si. O 2 is released. For example, Ca++ Plagioclases contain less silica than the K-spar s, so the reaction is K-spar = Plagioclase + Quartz. Often the quartz all goes bright/extinct at the same time, as if it is all the same crystal. Figure 3 -21. Myrmekite formed in plagioclase at the boundary with K-feldspar. Photographs courtesy © L. Collins. http: //www. csun. edu/~vcgeo 005