Ch 3 States of Matter Kinetic Theory of






























- Slides: 36

Ch. 3 – States of Matter

*Kinetic Theory of Matter w - an explanation of how particles in matter behave w Tiny, constantly moving atoms & particles make up all matter. w The kinetic energy (motion) of these particles increases as temperature increases.

Four States of Matter ö Solids wlow KE - particles vibrate but can’t move around wdefinite shape & volume

Four States of Matter ö Liquids whigher KE - particles can move around but are still close together w. No definite shape wdefinite volume

Four States of Matter ö Gases whigh KE - particles can separate and move throughout container w. No definite shape or volume

Four States of Matter ö *Plasma - a state of matter that consists of free-moving ions and electrons w very high KE - particles collide with enough energy to break into charged particles w gas-like, no definite shape or volume w stars, fluorescent light bulbs

Four States of Matter ö*Fluid – a nonsolid state of matter in which the atoms or molecules are free to move past each other, as in a gas or liquid

Energy ö *Energy – the ability to cause change; the capacity to do work.

Energy ö *Kinetic energy - energy in the form of motion

Energy ö *Temperature – a measure of how hot (or cold) something is; a measure of the average kinetic energy of the particles in an object.

Energy ö Thermal Energy – the total kinetic energy of a substance’s atoms.

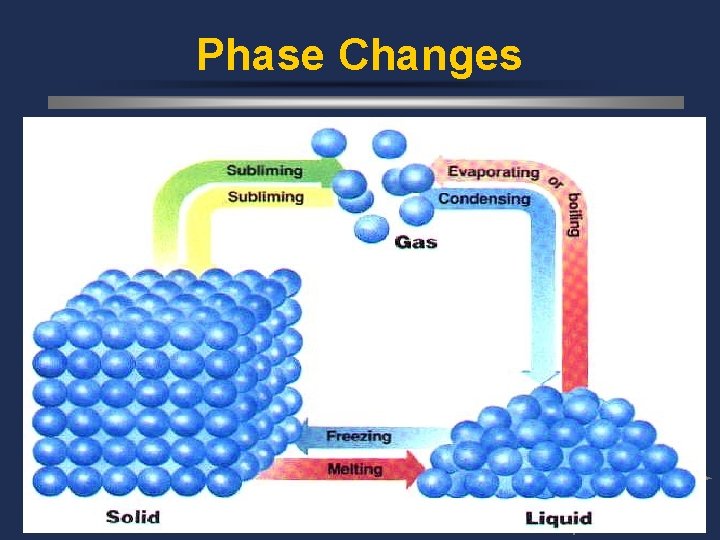
Phase Changes ö *Melting (for water, it’s 0°C) w solid to liquid ö *Freezing w liquid to solid melting point = freezing point

Phase Changes ö *Vaporization (boiling) w liquid to gas at the boiling point (for water, it’s 100°C) ö *Evaporation w liquid to gas below the boiling point ö *Condensation w gas to liquid

Phase Changes ö *Sublimation w solid to gas w EX: dry ice (CO 2), iodine

Phase Changes


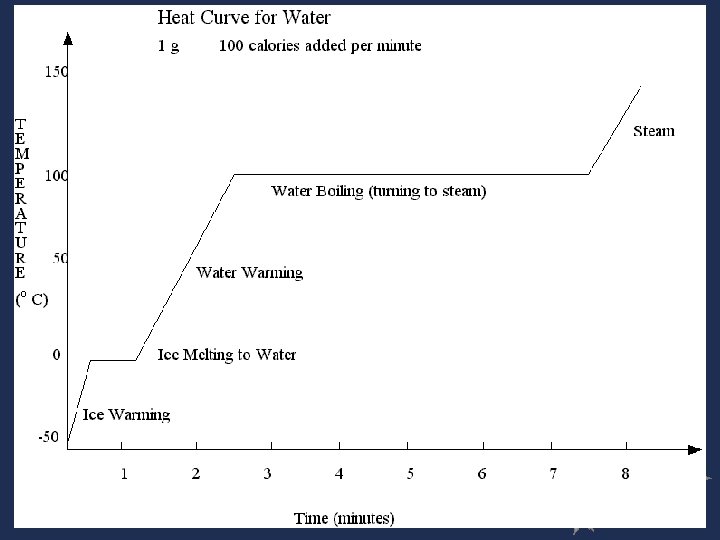
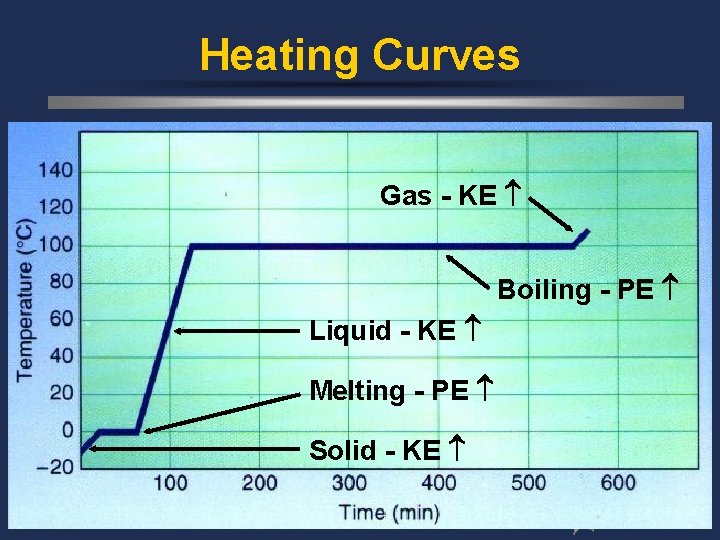
Heating Curves Gas - KE Boiling - PE Liquid - KE Melting - PE Solid - KE

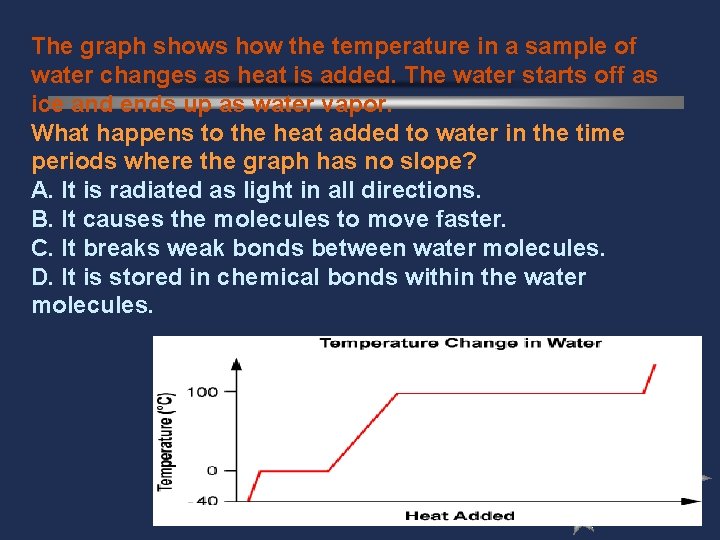
The graph shows how the temperature in a sample of water changes as heat is added. The water starts off as ice and ends up as water vapor. What happens to the heat added to water in the time periods where the graph has no slope? A. It is radiated as light in all directions. B. It causes the molecules to move faster. C. It breaks weak bonds between water molecules. D. It is stored in chemical bonds within the water molecules.

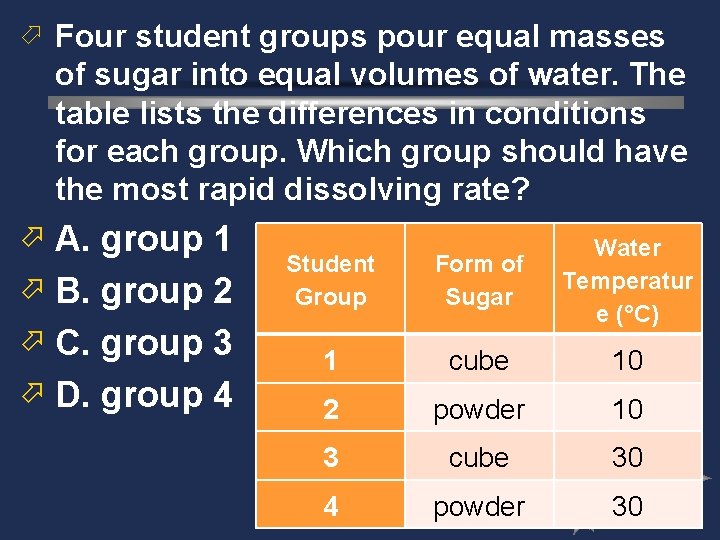
ö Four student groups pour equal masses of sugar into equal volumes of water. The table lists the differences in conditions for each group. Which group should have the most rapid dissolving rate? ö A. group 1 ö B. group 2 ö C. group 3 ö D. group 4 Student Group Form of Sugar Water Temperatur e (°C) 1 cube 10 2 powder 10 3 cube 30 4 powder 30

Law of conservation of mass – mass can be conserved; mass cannot be created or destroyed *Law of conservation of energy – energy can be conserved; energy cannot be created or destroyed

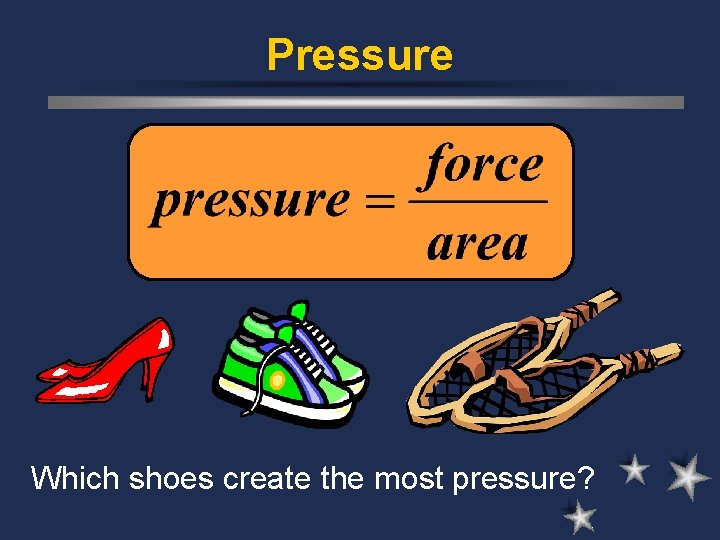
*Pressure ö – force exerted per unit area. ö *Pascal - the SI unit of pressure (Pa) ö Think about air pressure, blood pressure, water pressure ö Ex. Adding air to a bicycle tire

Pressure ö ö At sea level there is a pressure equivalent to 10 meters of water pressing down on all of us all the time. This is because of the weight of the air above us in the atmosphere. When you travel up a mountain, there is less air above you in the atmosphere, The important effect of this decrease in pressure is this: in a given volume of air, there are fewer molecules present. This is really just another way of saying that the pressure is lower. (This is called Boyle's law. ) The percentage of those molecules that are oxygen is exactly the same: 21%. The problem is that there are fewer molecules of everything present, including oxygen.

Pressure Which shoes create the most pressure?

Pascal’s Principle ö – A change in pressure at any point in an enclosed fluid will be transmitted equally to all parts of the fluid ö Ex. Squeezing a tube of toothpaste to make it come out. ö Ex. Squeezing one side of a balloon and the other end expands out.

*Buoyancy ö – the ability of a fluid (liquid or gas) to exert an upward force on an object immersed in it. ö If the buoyant force is equal to the object’s weight, the object will float. If the buoyant force is less than the object’s weight, the object will sink.

Archimedes’ Principle – the buoyant force on an object in a fluid is an upward force equal to the weight of the fluid that the object displaces. ö Ex. A cork floats because it is less dense than water. A piece of steel will sink because it is more dense than water.

Bernoulli’s Principle ö – as the speed of a moving fluid increases, the pressure of the moving fluid decreases ö Ex: Airplanes – The air flow on top of wing has less air pressure. The high pressure from underneath the wing pushes the wing up.

ö *Viscosity – a resistance to flow by a fluid. ö Ex. Syrup (especially if cold) will flow more slowly than water because syrup has a higher viscosity.

Gas Laws ö Gas Laws – the laws that state the mathematical relationships between the volume, temperature, pressure, and quantity of gas

Gas Laws Boyle’s Law – at constant temperature: the volume of a gas increases as the gas’s pressure decreases; or the volume of a gas decreases as the gas’s pressure increases

Boyle’s Law ö If you take the gas out of a large container and put it in a smaller container, the pressure will increase. Pressure of a gas depends on how often its particles strike the walls of the container. The particles will strike the walls more often in the smaller container, increasing the pressure.

Boyle’s Law ö The law also states as pressure is decreased, the volume is increased. ö Ex: As a balloon rises, the atmospheric pressure decreases. The volume of the balloon increases.

Gas Laws Gay-Lussac’s Law – at a constant volume: the pressure of a gas increases as the temperature decreases; the pressure of a gas increases as the temperature decreases

Charles’ Law ö Charles’s law – at a constant pressure: the volume of a gas increases as the gas’s temperature increases; or the volume of a gas decreases as the gas’s temperature decreases ö Ex. A car tire’s volume will decrease in the winter. A balloon will shrink in the cold.

*Thermal Expansion ö - an increase in the size of a substance when the temperature is increased ö Most matter expands when heated & contracts when cooled. ö Temp causes KE. Particles collide with more force & spread out.

ö Four student groups pour equal masses of sugar into equal volumes of water. The table lists the differences in conditions for each group. Which group should have the most rapid dissolving rate?