Ch 3 Notes Classifying Matter and its properties







- Slides: 25

Ch. 3 Notes Classifying Matter and it’s properties

Objectives �Introduce Chemistry �SWBAT explain the differences between the TWO major classifications of matter �SWBAT explain some of the similarities and differences of the properties of these groups

Chemistry �The study of the composition, structure, properties, and reactions of MATTER.

Matter �A physical substance. �It occupies space (has a volume) �It has a mass.

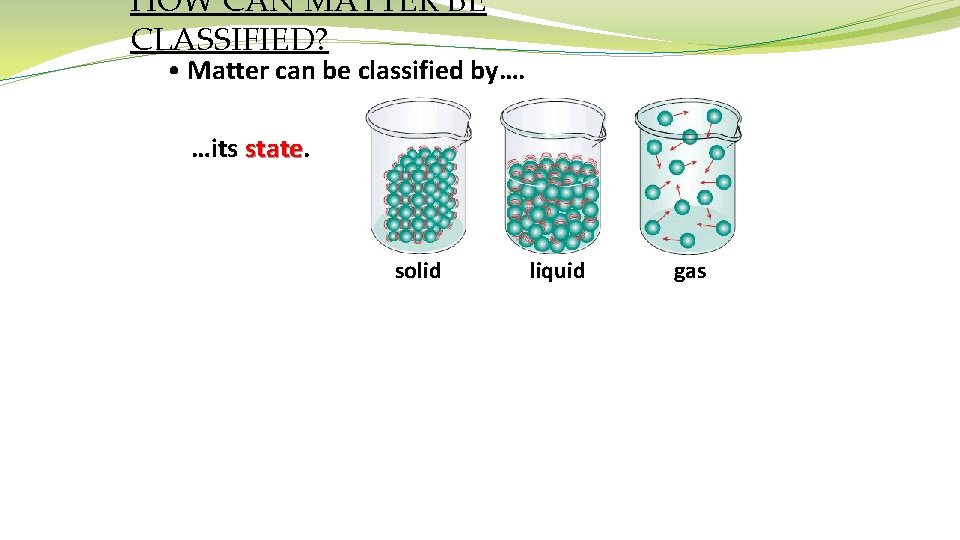
HOW CAN MATTER BE CLASSIFIED? • Matter can be classified by…. …its state solid liquid gas

HOW CAN MATTER BE CLASSIFIED? • Matter can be classified by…. …its state …its color

HOW CAN MATTER BE CLASSIFIED? • Matter can be classified by…. …its state …its color …its uses

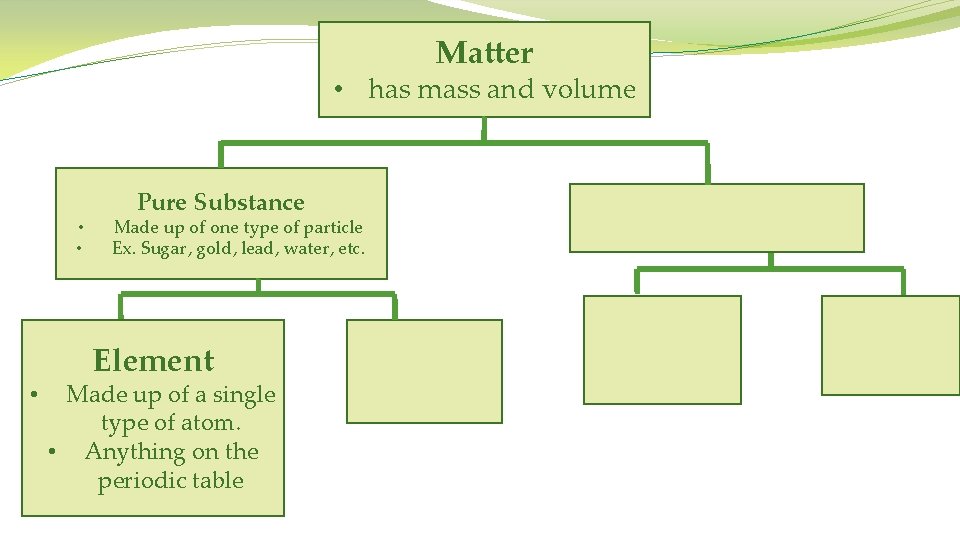
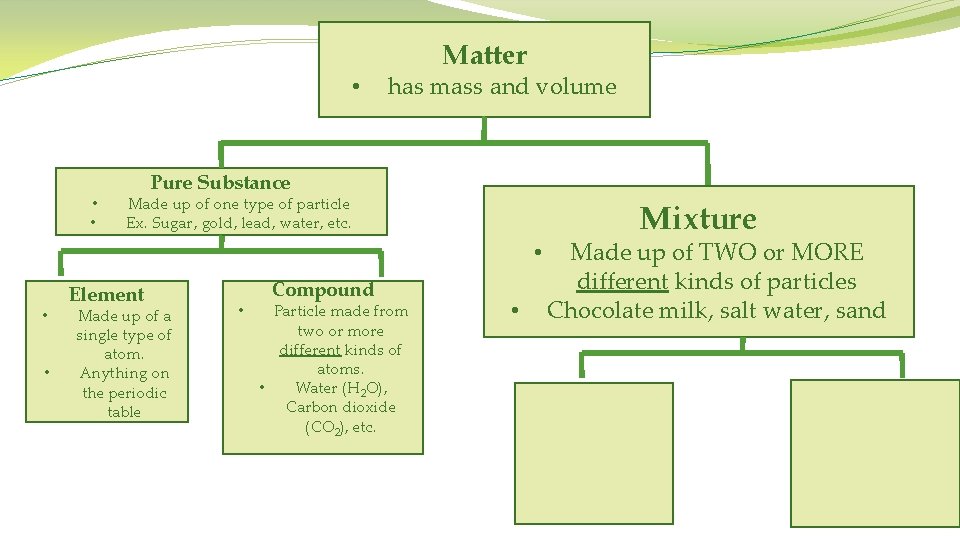
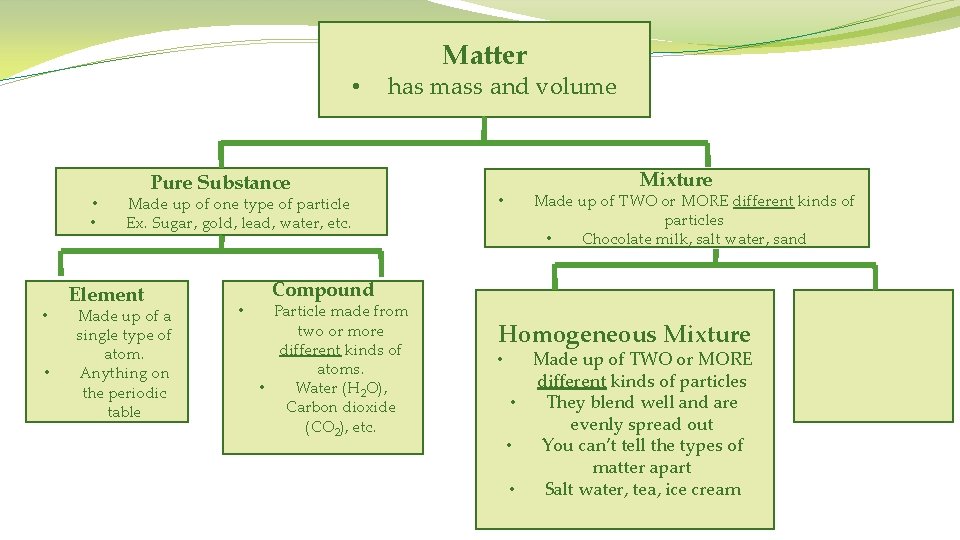
Matter • has mass and volume • • Pure Substance Made up of one type of particle Ex. Sugar, gold, lead, water, etc.

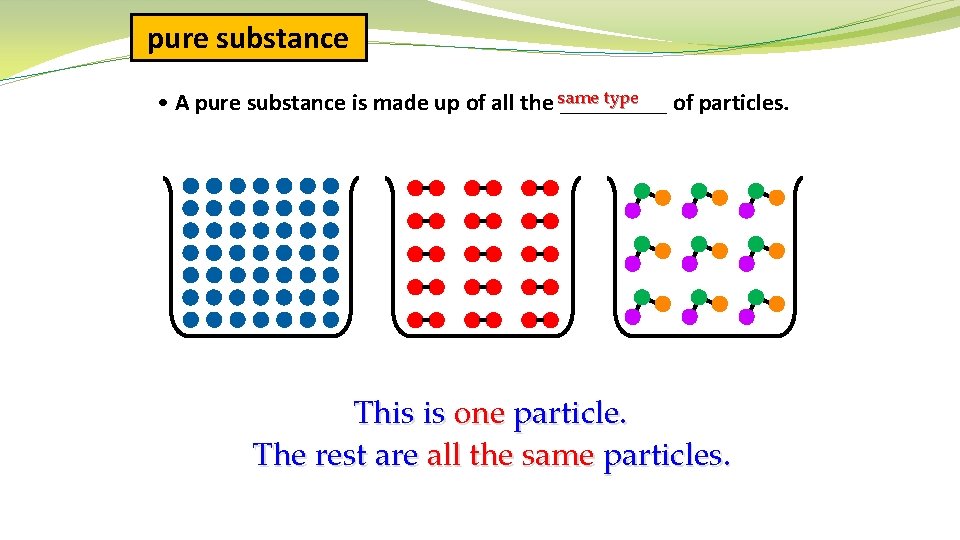
pure substance type • A pure substance is made up of all the same _____ of particles. This is one particle. The rest are all the same particles.

Matter • has mass and volume • • • Pure Substance Made up of one type of particle Ex. Sugar, gold, lead, water, etc. Element Made up of a single type of atom. • Anything on the periodic table

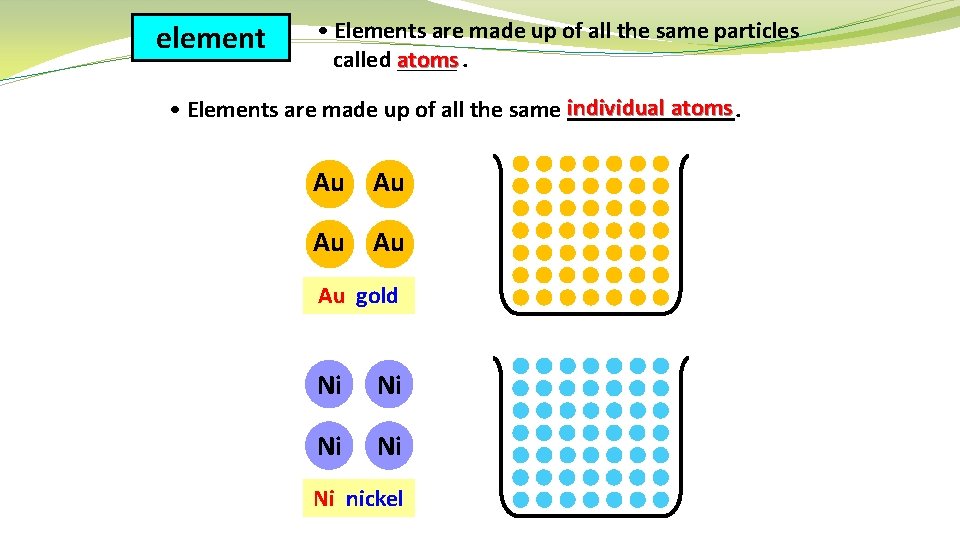
element • Elements are made up of all the same particles called atoms _____. atoms • Elements are made up of all the same individual _______. Au Au Au gold Ni Ni Ni nickel

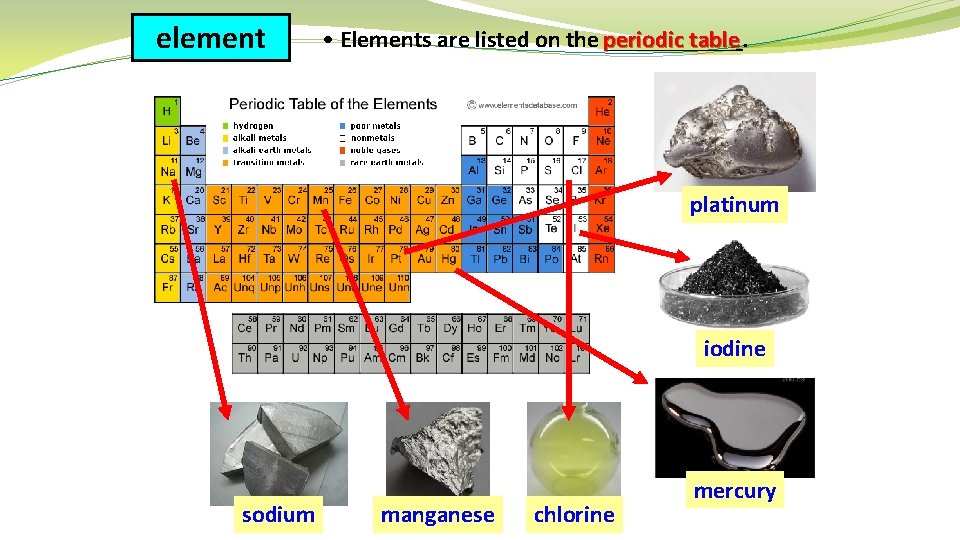
element table. • Elements are listed on the periodic ______ platinum iodine sodium manganese chlorine mercury

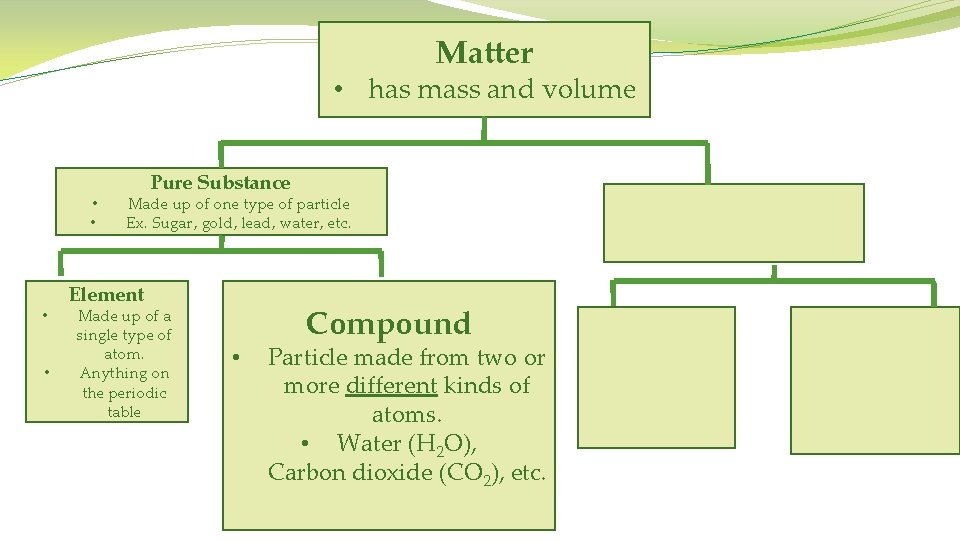
Matter • has mass and volume • • Pure Substance Made up of one type of particle Ex. Sugar, gold, lead, water, etc. Element Made up of a single type of atom. Anything on the periodic table • Compound Particle made from two or more different kinds of atoms. • Water (H 2 O), Carbon dioxide (CO 2), etc.

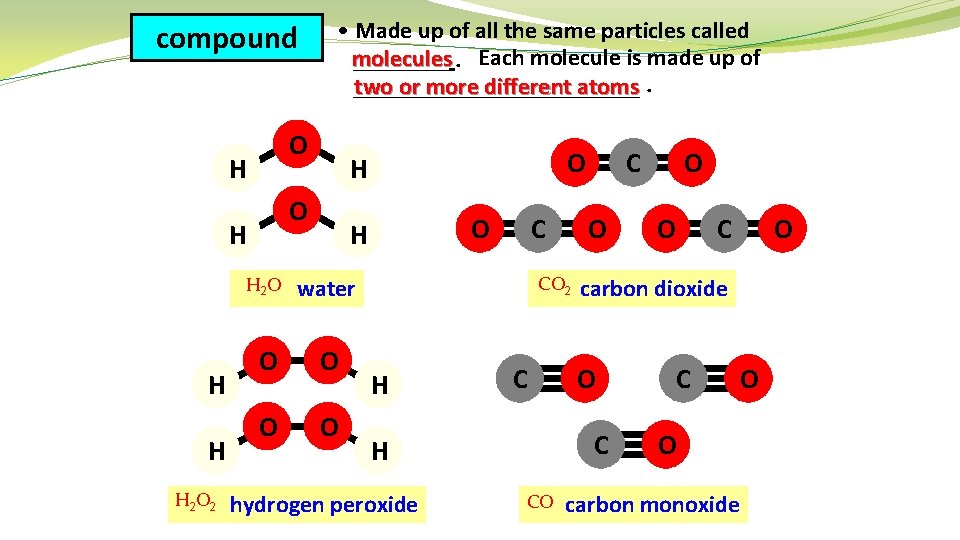
compound O H H H 2 O H O H H • Made up of all the same particles called molecules ____. Each molecule is made up of ____________ two or more different atoms. O H H 2 O water O O O C CO 2 H C hydrogen peroxide CO O O C carbon dioxide O C H O C C O carbon monoxide O

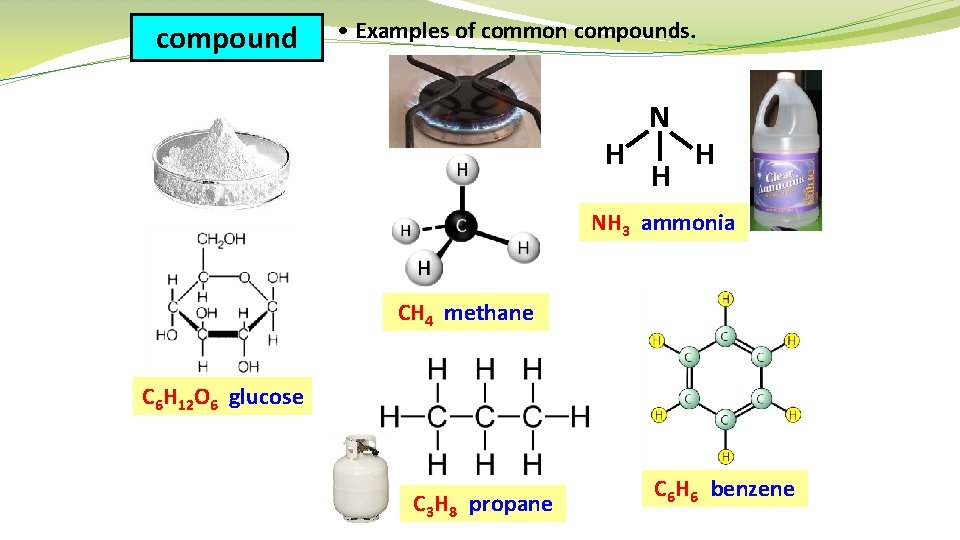
compound • Examples of common compounds. H N H H NH 3 ammonia CH 4 methane C 6 H 12 O 6 glucose C 3 H 8 propane C 6 H 6 benzene

• • • Matter has mass and volume Pure Substance Made up of one type of particle Ex. Sugar, gold, lead, water, etc. • • • Element Made up of a single type of atom. Anything on the periodic table • Compound Particle made from two or more different kinds of atoms. • Water (H 2 O), Carbon dioxide (CO 2), etc. • Mixture Made up of TWO or MORE different kinds of particles Chocolate milk, salt water, sand

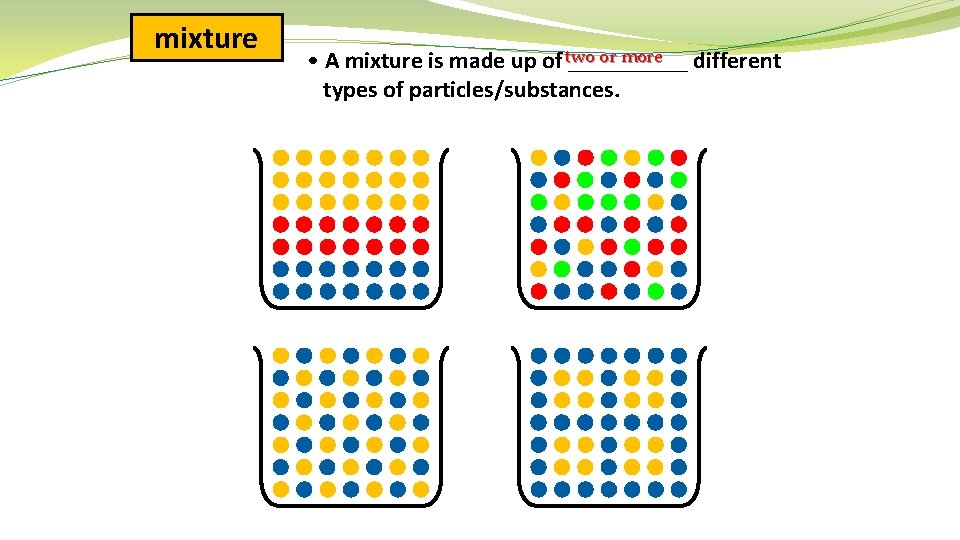
mixture or more • A mixture is made up of two _____ different types of particles/substances.

• • • has mass and volume Pure Substance Made up of one type of particle Ex. Sugar, gold, lead, water, etc. Element Made up of a single type of atom. Anything on the periodic table • Matter • Mixture Made up of TWO or MORE different kinds of particles • Chocolate milk, salt water, sand Compound Particle made from two or more different kinds of atoms. • Water (H 2 O), Carbon dioxide (CO 2), etc. Homogeneous Mixture • Made up of TWO or MORE different kinds of particles • They blend well and are evenly spread out • You can’t tell the types of matter apart • Salt water, tea, ice cream

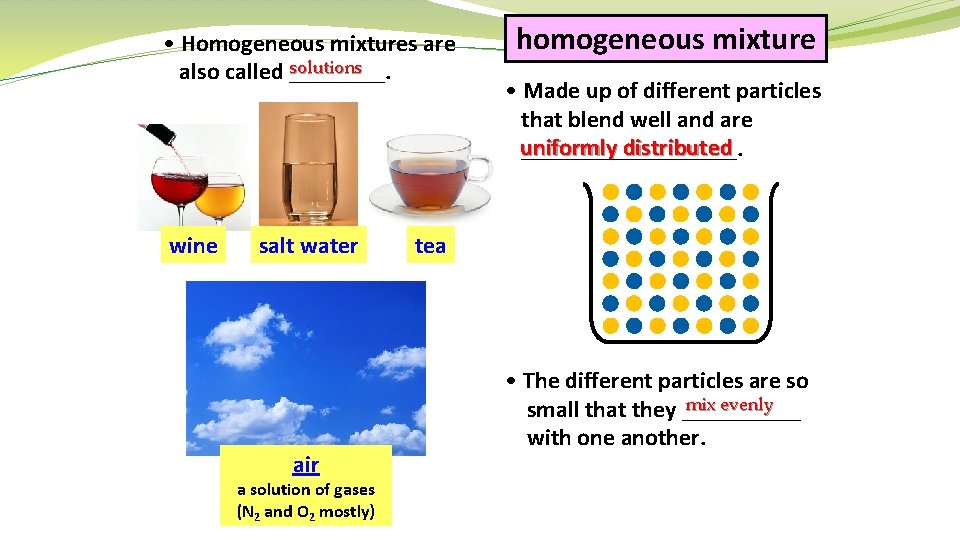
• Homogeneous mixtures are solutions also called ____. wine salt water air a solution of gases (N 2 and O 2 mostly) homogeneous mixture • Made up of different particles that blend well and are uniformly distributed _________. tea • The different particles are so mix evenly small that they _____ with one another.

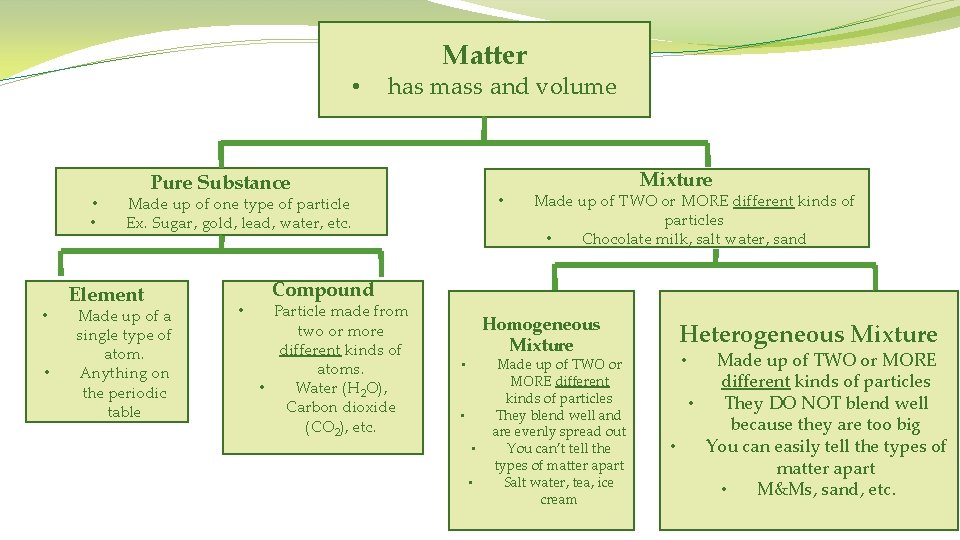
• • • Matter has mass and volume Pure Substance • Made up of one type of particle Ex. Sugar, gold, lead, water, etc. Element Made up of a single type of atom. Anything on the periodic table • Mixture Made up of TWO or MORE different kinds of particles • Chocolate milk, salt water, sand Compound Particle made from two or more different kinds of atoms. • Water (H 2 O), Carbon dioxide (CO 2), etc. Homogeneous Mixture • • Made up of TWO or MORE different kinds of particles They blend well and are evenly spread out You can’t tell the types of matter apart Salt water, tea, ice cream Heterogeneous Mixture Made up of TWO or MORE different kinds of particles • They DO NOT blend well because they are too big • You can easily tell the types of matter apart • M&Ms, sand, etc. •

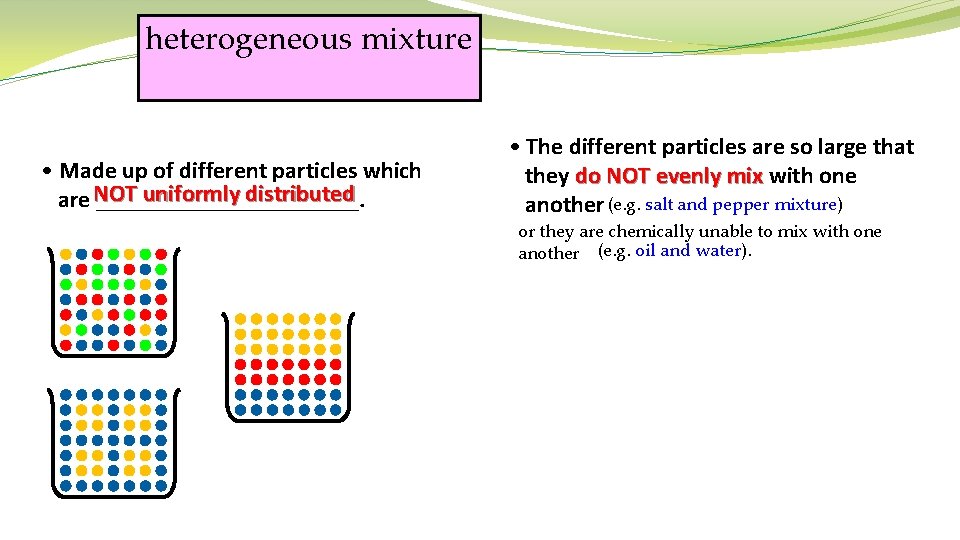
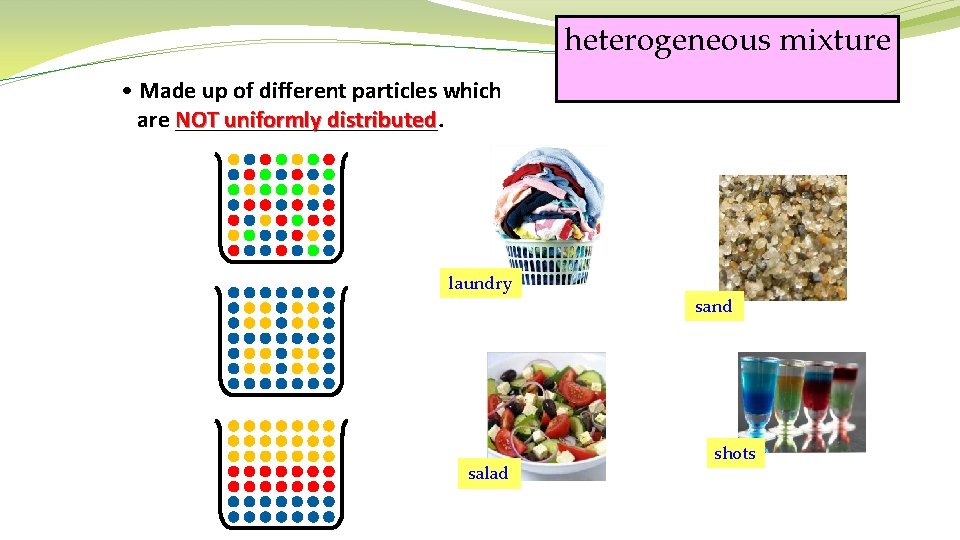
heterogeneous mixture • Made up of different particles which uniformly distributed are NOT ___________. • The different particles are so large that they do NOT evenly mix with one another (e. g. salt and pepper mixture) or they are chemically unable to mix with one another (e. g. oil and water).

heterogeneous mixture • Made up of different particles which are NOT ___________. uniformly distributed laundry salad sand shots

Special Types of Mixtures �Solution – a homogeneous mixture where one substance dissolves completely into another, and looks uniform throughout. The substances cannot be separated easily �Ex. Sugar and water, salt and water Sugar Water Solution

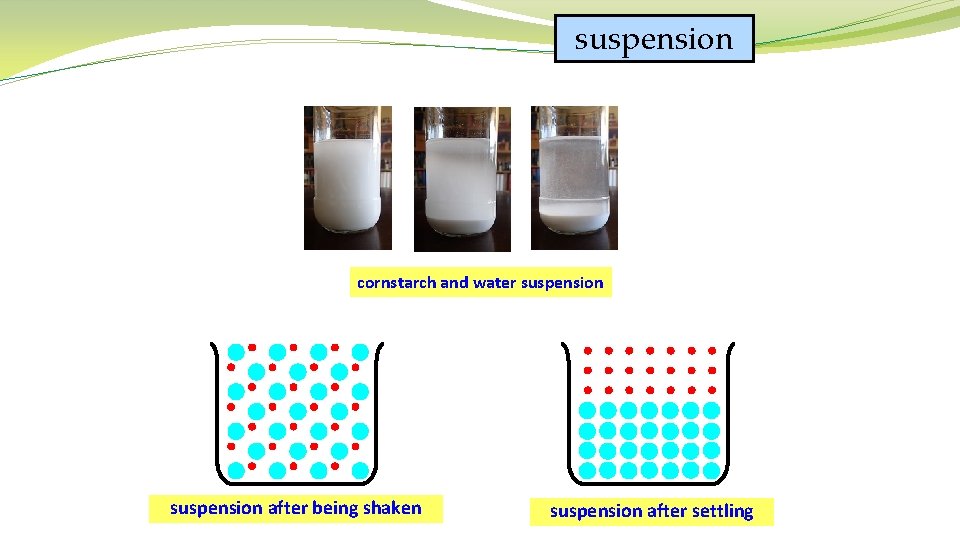
�Suspension – a heterogeneous mixture that will separate into layers over time. Its parts can be separated fairly easily. �Ex. Sand in water. Drinks that say “shake well”

suspension cornstarch and water suspension after being shaken suspension after settling