Ch 3 Atomic Structure II Subatomic Particles Development
Ch. 3 - Atomic Structure II. Subatomic Particles
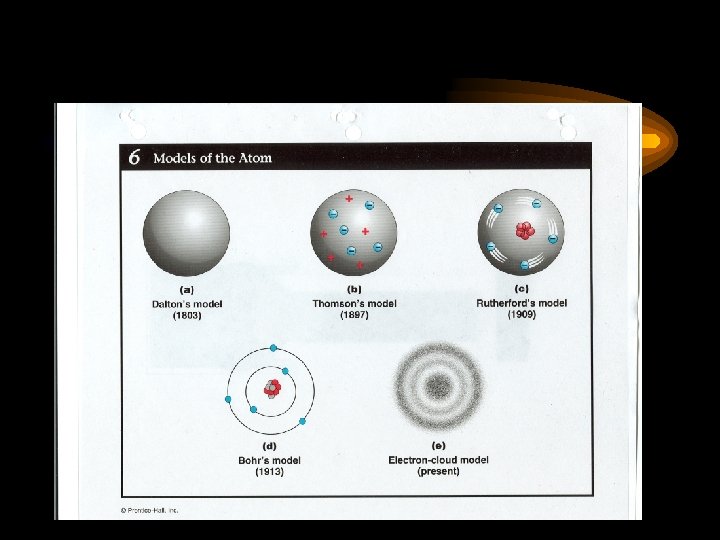

Development of the Atom 1 st Model: Dalton’s Theory (p. 78) 1. All matter is made of indivisible atoms. 2. All atoms of a given element are identical. 2 nd Model: Thomson’s model (p. 82, p. 84) “Plum-pudding” model. Discovered electron (negative charges) through Cathode Ray Tube Experiment. 3 rd Model: Rutherford’s Model (p. 84) Discovered the nucleus made of protons through Gold Foil Experiment.
Development of the Atom ¨ 4 th Model: Bohr’s Model (p. 96) “Planetary” model: Electrons orbit the nucleus on specific paths. ¨ Chadwick: (p. 86, p. 89) Discovered the neutrons in the nucleus. Neutrons are the “glue” of the atom, or strong nuclear force.
Development of the Atom ¨ 5 th Model: Electron Cloud Model, the current model of the atom. Developed by Schrodinger (p. 96). Electrons are in “clouds” or regions of space outside the nucleus. Also called orbitals or energy levels.
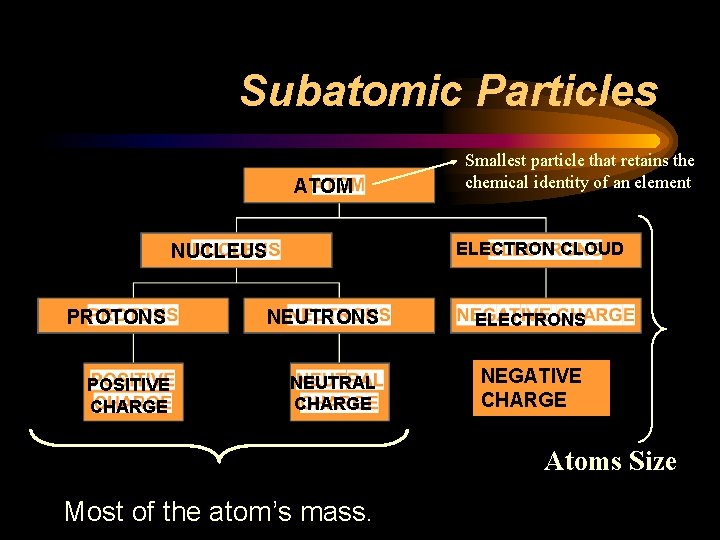
Subatomic Particles ATOM Smallest particle that retains the chemical identity of an element ELECTRON CLOUD NUCLEUS PROTONS NEUTRONS ELECTRONS POSITIVE CHARGE NEUTRAL CHARGE NEGATIVE CHARGE Atoms Size Most of the atom’s mass.

Electron Cloud ¨ The electron cloud is made of energy levels. • 1 st energy level: 2 e • 2 nd energy level: 8 e • 3 rd energy level: 18 e • And so on….

Subatomic particles ¨ Atomic Number (Moseley): the # of protons in the nucleus of an atom • Unique to each element • For example: Oxygen: Atomic # 8 8 protons 8 electrons Positive charge = Negative charge in a neutral atom
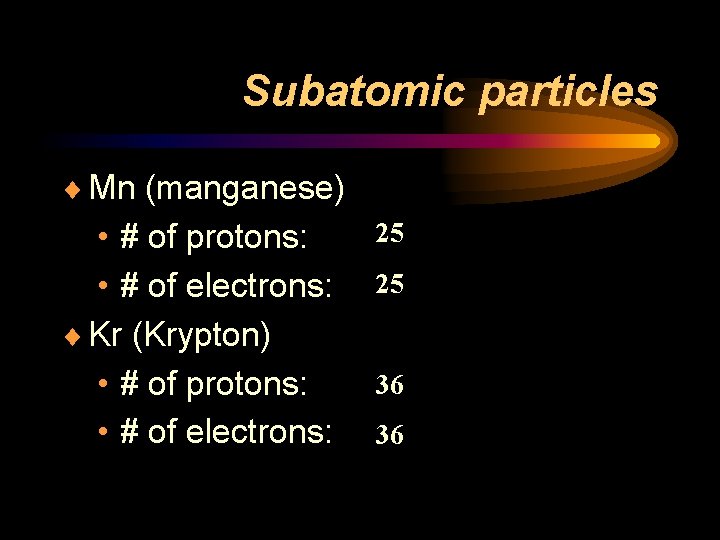
Subatomic particles ¨ Mn (manganese) • # of protons: • # of electrons: ¨ Kr (Krypton) • # of protons: • # of electrons: 25 25 36 36
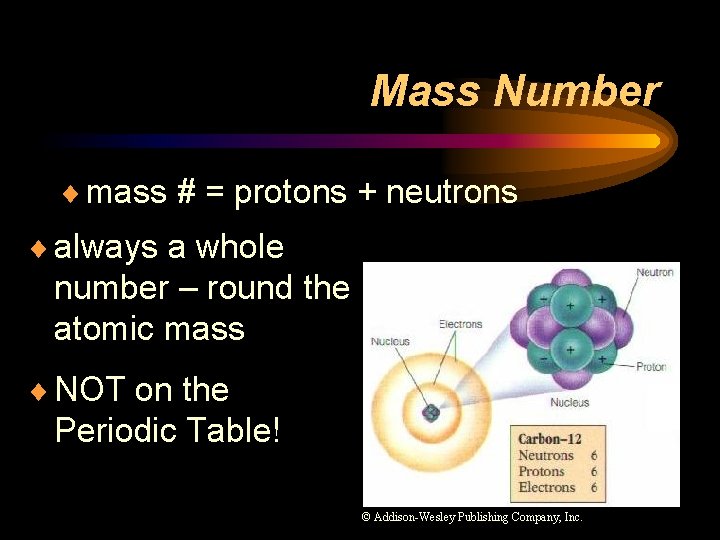
Mass Number ¨ mass # = protons + neutrons ¨ always a whole number – round the atomic mass ¨ NOT on the Periodic Table! © Addison-Wesley Publishing Company, Inc.
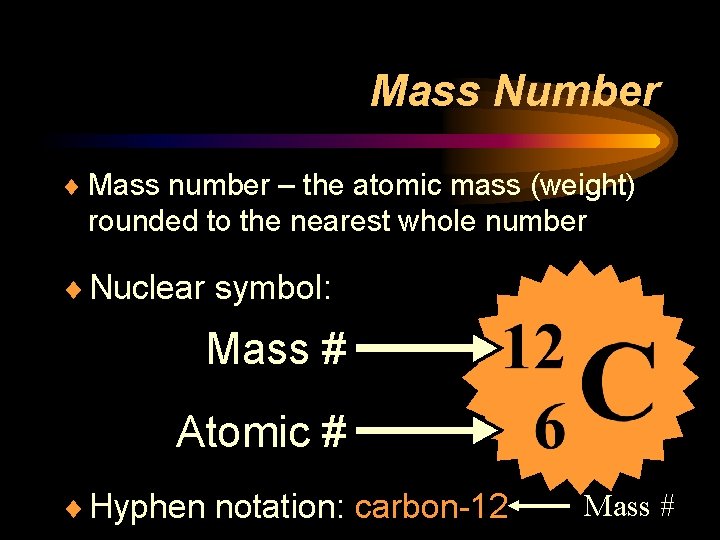
Mass Number ¨ Mass number – the atomic mass (weight) rounded to the nearest whole number ¨ Nuclear symbol: Mass # Atomic # ¨ Hyphen notation: carbon-12 Mass #
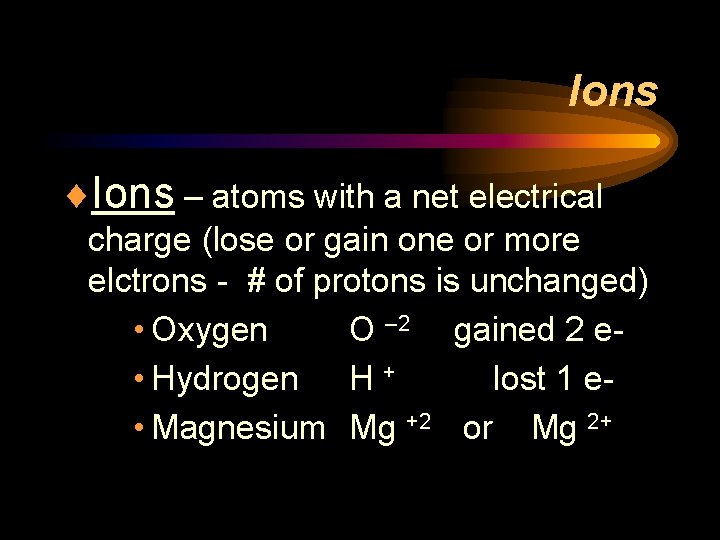
Ions ¨Ions – atoms with a net electrical charge (lose or gain one or more elctrons - # of protons is unchanged) • Oxygen O – 2 gained 2 e • Hydrogen H + lost 1 e • Magnesium Mg +2 or Mg 2+
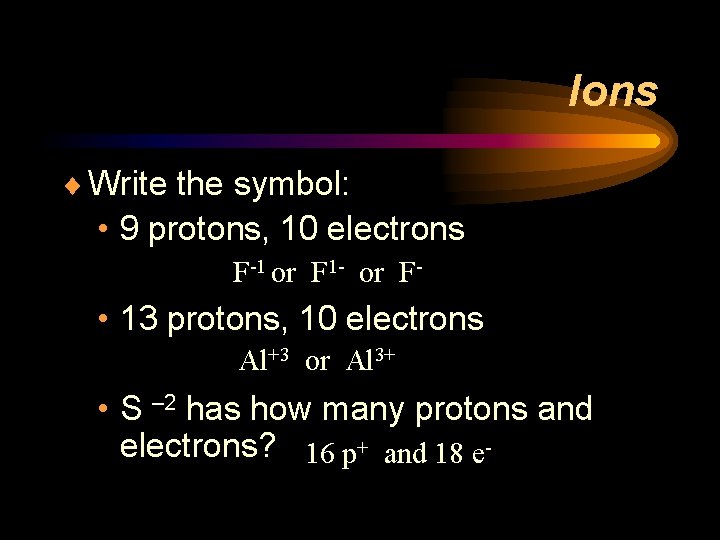
Ions ¨ Write the symbol: • 9 protons, 10 electrons F-1 or F 1 - or F- • 13 protons, 10 electrons Al+3 or Al 3+ • S – 2 has how many protons and electrons? 16 p+ and 18 e-

Ions ¨ Iron has how many protons, electrons and neutrons? 56 Fe 26 26 protons, 26 electrons, 30 neutrons ¨ Fe+2 has how many protons, electrons, and neutrons? 26 protons, 24 electrons, 30 neutrons

Isotopes … Atoms of the same element with different mass numbers ( diff. # of neutrons ). They don’t have to be radioactive but some isotopes are unstable and decay. However, there are many stable isotopes that don’t decay.
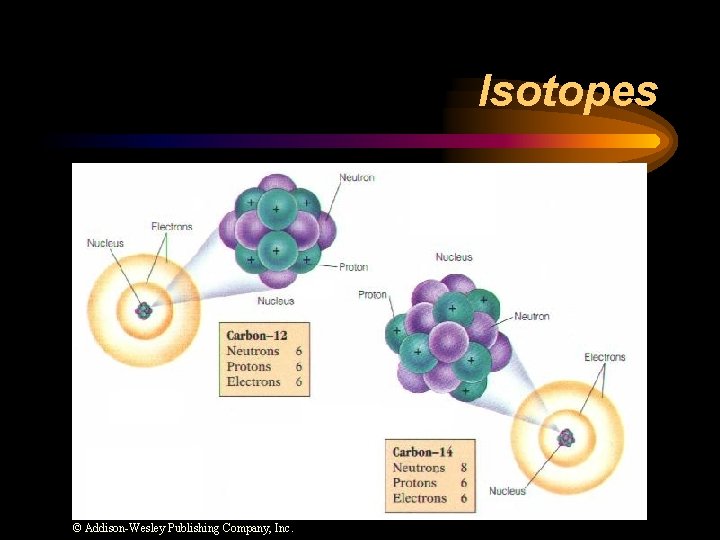
Isotopes © Addison-Wesley Publishing Company, Inc.
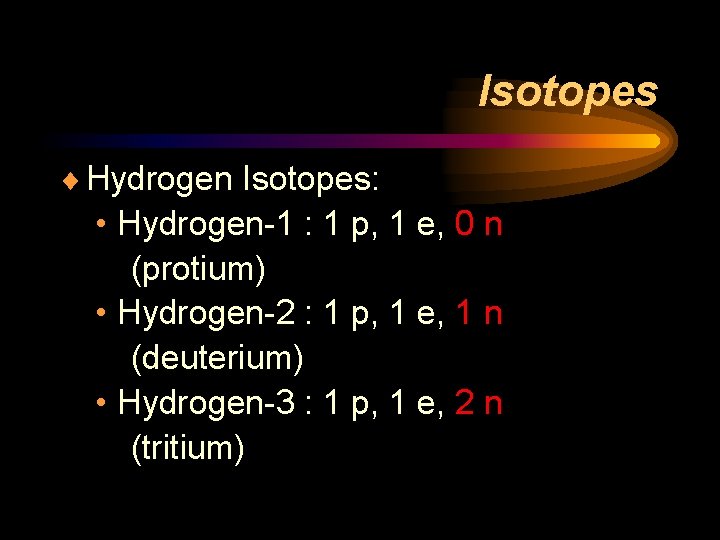
Isotopes ¨ Hydrogen Isotopes: • Hydrogen-1 : 1 p, 1 e, 0 n (protium) • Hydrogen-2 : 1 p, 1 e, 1 n (deuterium) • Hydrogen-3 : 1 p, 1 e, 2 n (tritium)

Isotopes Some elements have several Isotopes Lead has four naturally occurring isotopes, Pb-204, Pb-206, Pb-207, and Pb-208; but there are 23 manmade isotopes of lead.

Isotopes ¨ Chlorine-37 Write the nuclear symbol then determine: • atomic #: 17 • mass #: 37 • # of protons: 17 • # of electrons: 17 • # of neutrons: 20

Day 2 start here: Isotopes ¨ The periodic table gives the average atomic mass of all the naturally occurring isotopes according to how abundant the isotope is. ¨ If the avg. atomic mass is rounded to the nearest whole number it will tell the most abundant isotope. • Carbon: 12. 0107 amu so…… • Hydrogen: 1. 00794 amu so….

Relative Atomic Mass ¨ 12 C atom = 1. 992 × 10 -23 g ¨ atomic mass unit (amu – Symbol: ) ¨ 1 amu = 1/12 the mass of a 12 C atom ¨ 1 amu = 1. 66 × 10 -24 g ¨ Carbon: 12. 0107 amu ¨ NOT 12. 0107 g !! © Addison-Wesley Publishing Company, Inc.
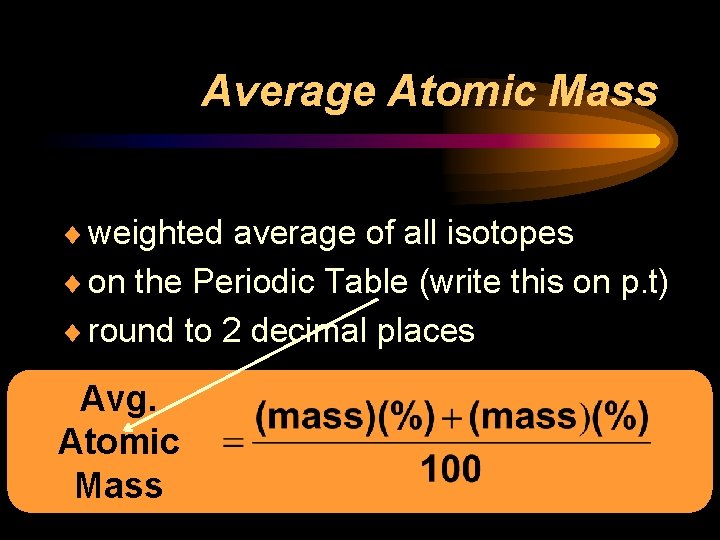
Average Atomic Mass ¨ weighted average of all isotopes ¨ on the Periodic Table (write this on p. t) ¨ round to 2 decimal places Avg. Atomic Mass
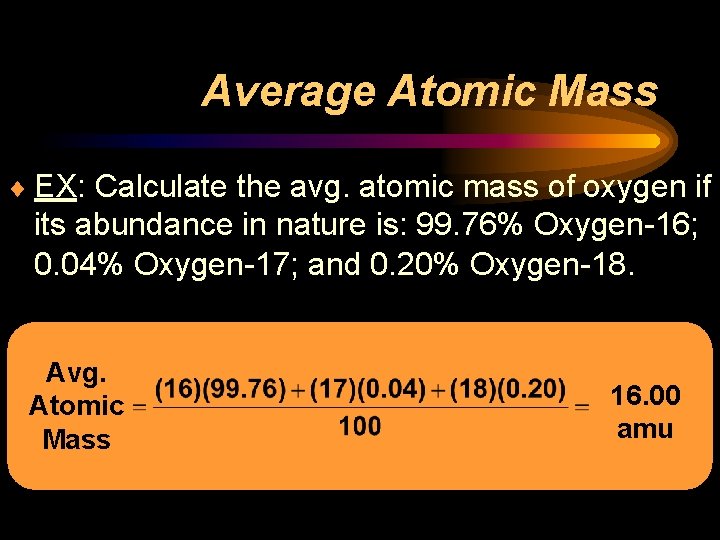
Average Atomic Mass ¨ EX: Calculate the avg. atomic mass of oxygen if its abundance in nature is: 99. 76% Oxygen-16; 0. 04% Oxygen-17; and 0. 20% Oxygen-18. Avg. Atomic Mass 16. 00 amu
- Slides: 23