CH 3 4 Predicting acid strength Periodic Table
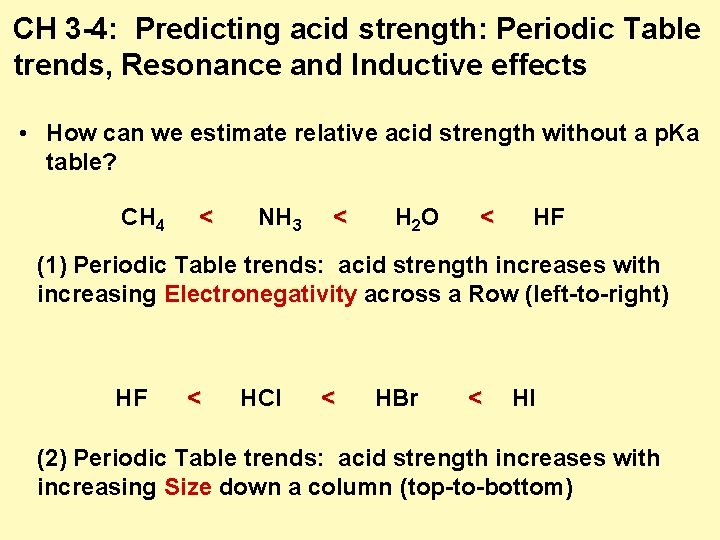
CH 3 -4: Predicting acid strength: Periodic Table trends, Resonance and Inductive effects • How can we estimate relative acid strength without a p. Ka table? CH 4 < NH 3 < H 2 O < HF (1) Periodic Table trends: acid strength increases with increasing Electronegativity across a Row (left-to-right) HF < HCl < HBr < HI (2) Periodic Table trends: acid strength increases with increasing Size down a column (top-to-bottom)
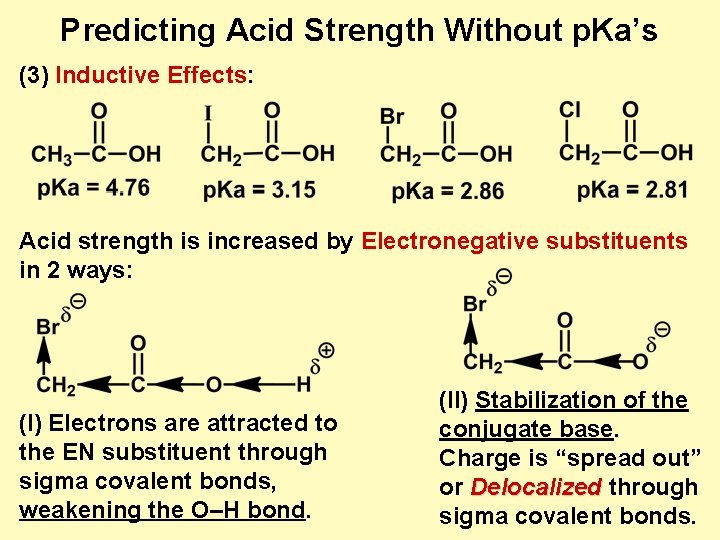
Predicting Acid Strength Without p. Ka’s (3) Inductive Effects: Acid strength is increased by Electronegative substituents in 2 ways: (I) Electrons are attracted to the EN substituent through sigma covalent bonds, weakening the O–H bond. (II) Stabilization of the conjugate base. Charge is “spread out” or Delocalized through sigma covalent bonds.
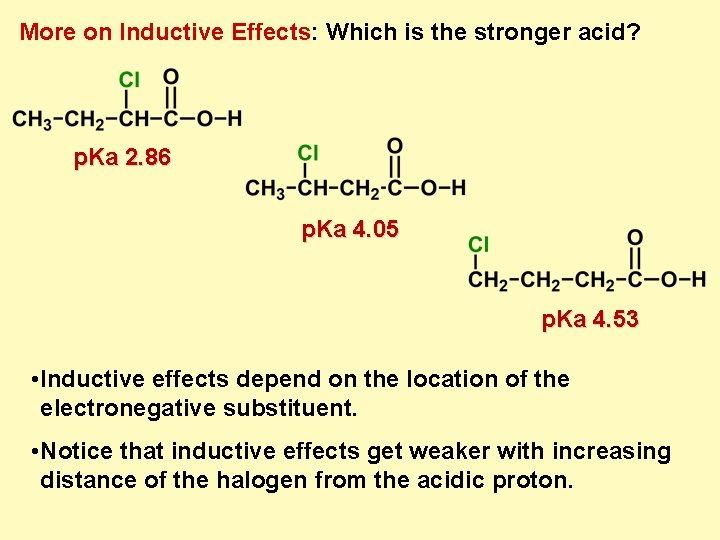
More on Inductive Effects: Which is the stronger acid? p. Ka 2. 86 p. Ka 4. 05 p. Ka 4. 53 • Inductive effects depend on the location of the electronegative substituent. • Notice that inductive effects get weaker with increasing distance of the halogen from the acidic proton.
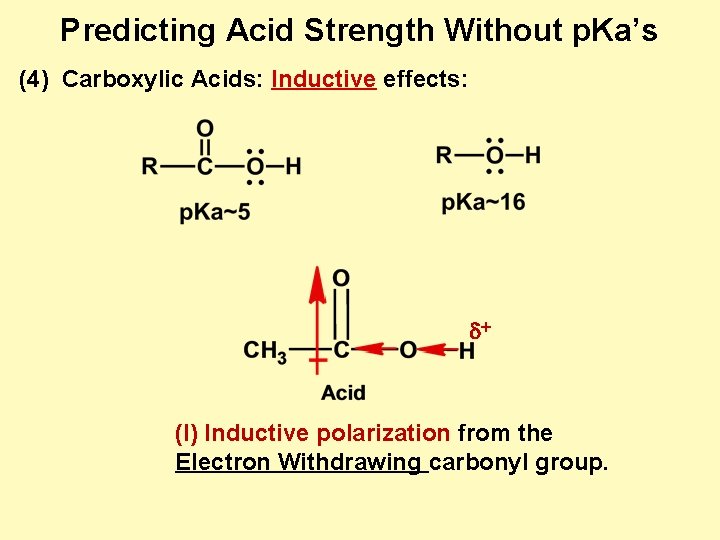
Predicting Acid Strength Without p. Ka’s (4) Carboxylic Acids: Inductive effects: d+ (I) Inductive polarization from the Electron Withdrawing carbonyl group.
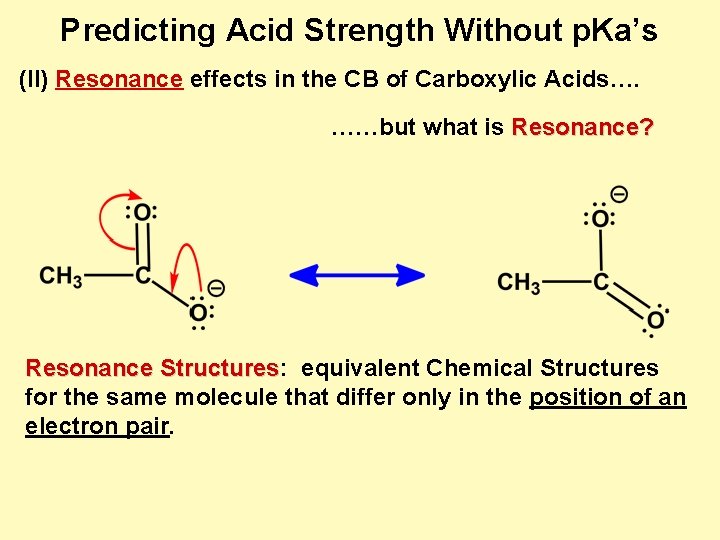
Predicting Acid Strength Without p. Ka’s (II) Resonance effects in the CB of Carboxylic Acids…. ……but what is Resonance? Resonance Structures: Structures equivalent Chemical Structures for the same molecule that differ only in the position of an electron pair.
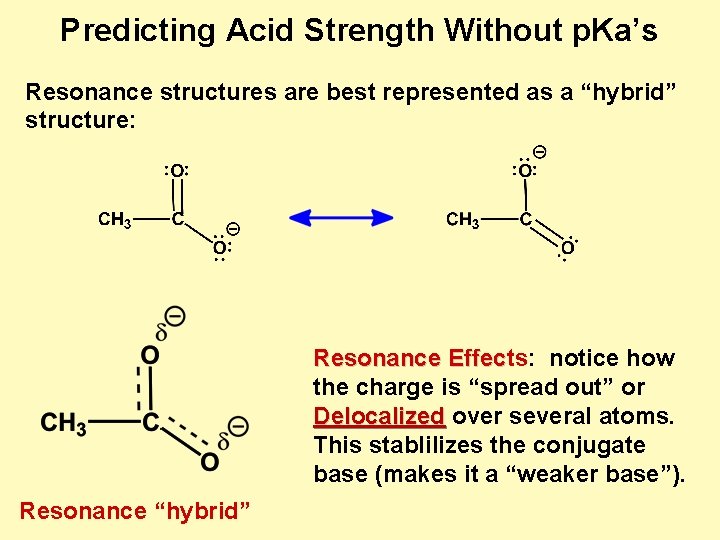
Predicting Acid Strength Without p. Ka’s Resonance structures are best represented as a “hybrid” structure: Resonance Effects: notice how Effec the charge is “spread out” or Delocalized over several atoms. This stablilizes the conjugate base (makes it a “weaker base”). Resonance “hybrid”
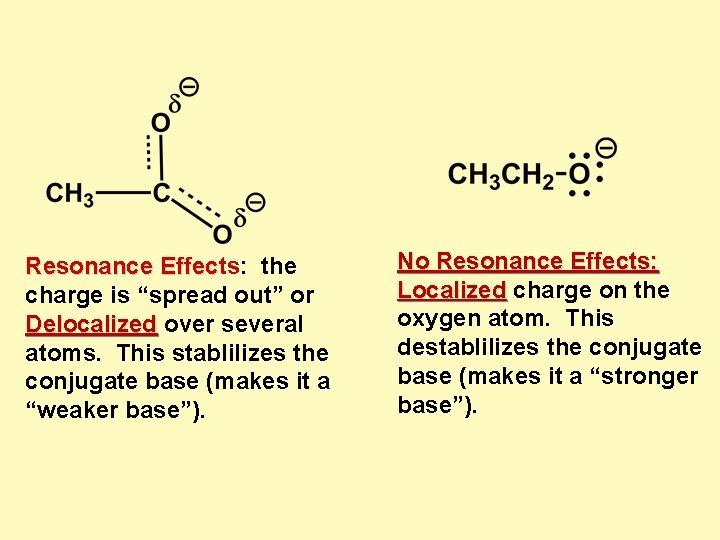
Resonance Effects: the Effec charge is “spread out” or Delocalized over several atoms. This stablilizes the conjugate base (makes it a “weaker base”). No Resonance Effects: Localized charge on the oxygen atom. This destablilizes the conjugate base (makes it a “stronger base”).
- Slides: 7