Ch 235 42 Advanced Analytical Chemistry NMR Rene






- Slides: 35

Ch 235. 42: Advanced Analytical Chemistry: NMR Rene Angelo S. Macahig, Ph. D 2 nd Sem AY 2012 -2013

COURSE OBJECTIVES 1. Understand theoretical basis of NMR; 2. Use of NMR for organic compounds and to observe other nuclei, such as 31 P or 19 F 3. Understand common 1 D and 2 D NMR pulse sequences 4. Basic principles of NMR instrumentation; 5. Operate the NMR instrument properly and develop sufficient skill to be an NMR operator; and 6. Interpret NMR spectra of organic compounds.

Spectroscopy: The study of molecular structure and dynamics through the absorption, emission and scattering of light. (www. science. marshall. edu)

(apwww. smu. ca/~ishort/Astro/spectroscopy. gif)

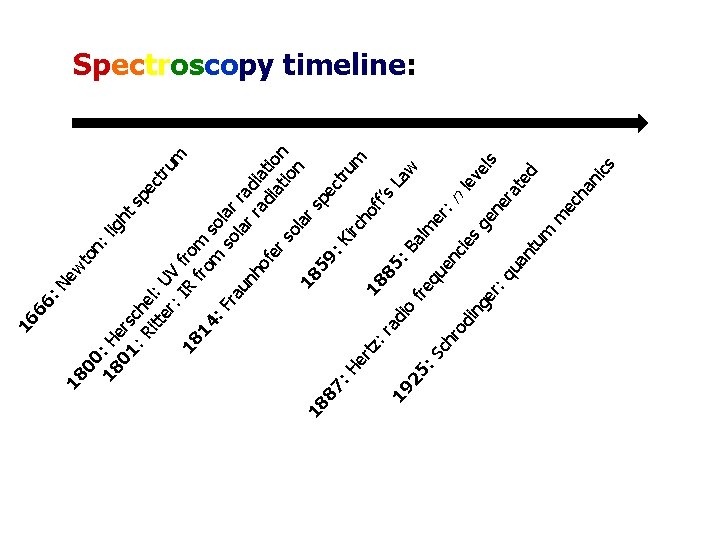
to ew n n: 18 : H lig ht 01 ers c sp : R he ec itt l: tru er UV : I f m 18 R ro fro m 14 m so : F so lar ra la ra un rr d ho ad iat fe ia ion rs tio ol n ar 1 85 18 sp 9: 87 ec tru : H Ki rc m er h of tz 18 : r f’s 85 a La 19 di : B o w 25 al fre m : S qu er ch en : ro c ie le di s ve ng ge ls er ne : q ra ua te nt d um m ec ha ni cs 00 18 : N 66 16 Spectroscopy timeline:

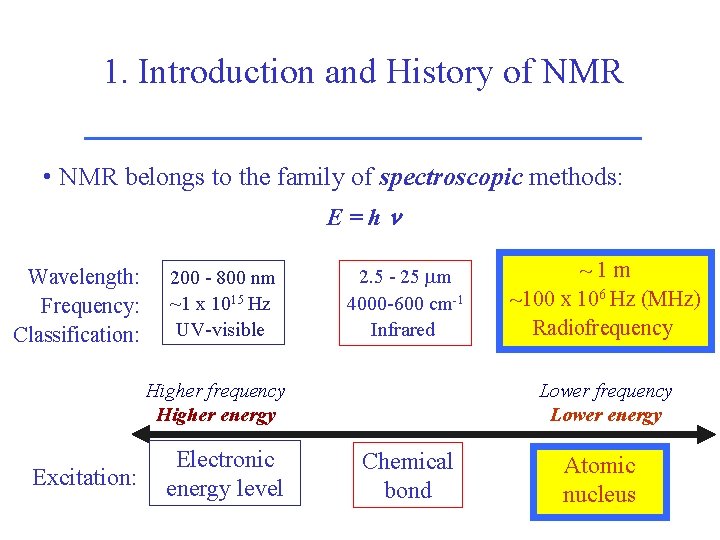
1. Introduction and History of NMR • NMR belongs to the family of spectroscopic methods: E=hn Wavelength: Frequency: Classification: 200 - 800 nm ~1 x 1015 Hz UV-visible 2. 5 - 25 m 4000 -600 cm-1 Infrared Higher frequency Higher energy Excitation: Electronic energy level ~1 m ~100 x 106 Hz (MHz) Radiofrequency Lower energy Chemical bond Atomic nucleus

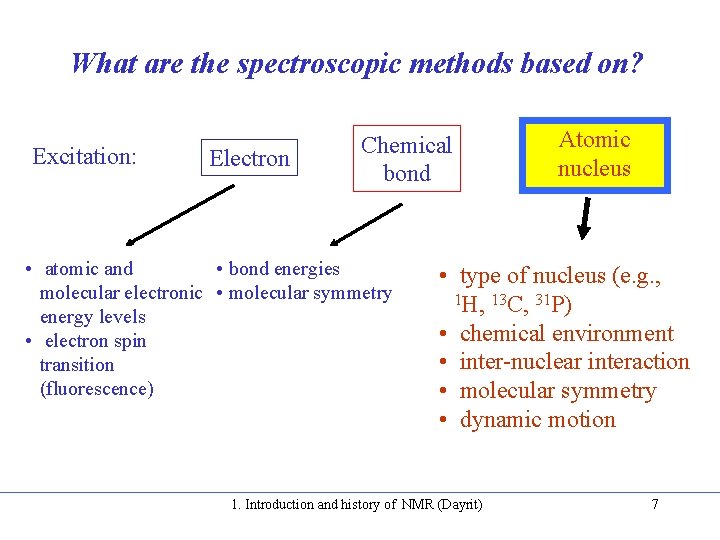
What are the spectroscopic methods based on? Excitation: Electron Chemical bond • atomic and • bond energies molecular electronic • molecular symmetry energy levels • electron spin transition (fluorescence) Atomic nucleus • type of nucleus (e. g. , 1 H, 13 C, 31 P) • chemical environment • inter-nuclear interaction • molecular symmetry • dynamic motion 1. Introduction and history of NMR (Dayrit) 7

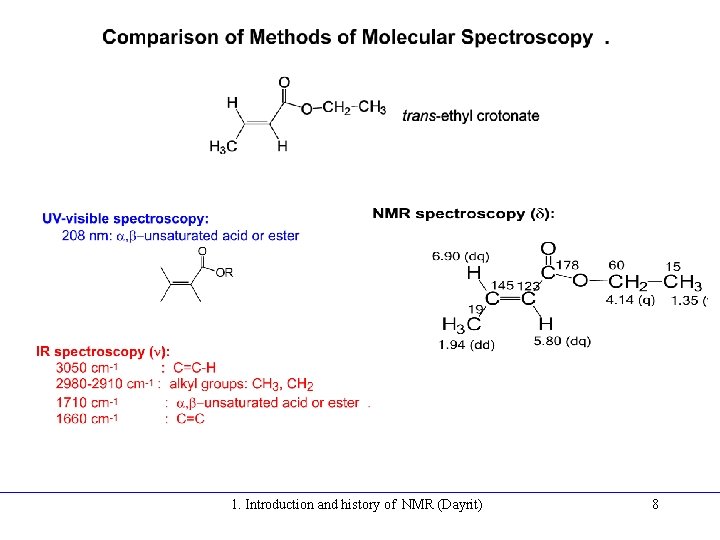
1. Introduction and history of NMR (Dayrit) 8

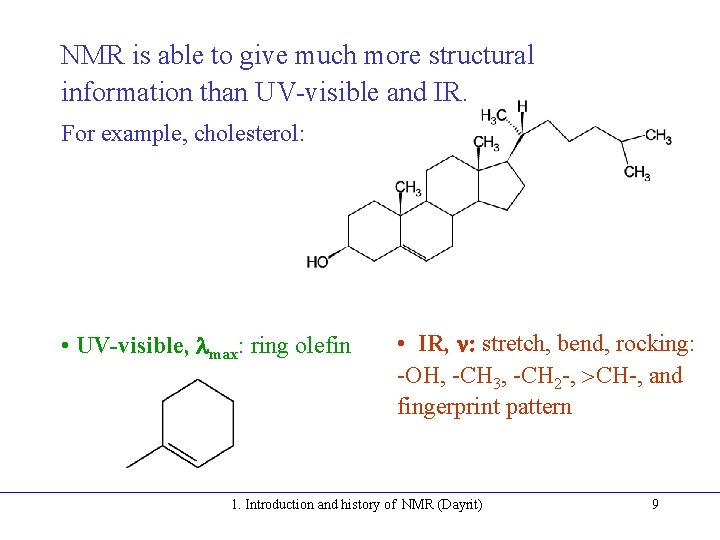
NMR is able to give much more structural information than UV-visible and IR. For example, cholesterol: • UV-visible, lmax: ring olefin • IR, n: stretch, bend, rocking: -OH, -CH 3, -CH 2 -, CH-, and fingerprint pattern 1. Introduction and history of NMR (Dayrit) 9

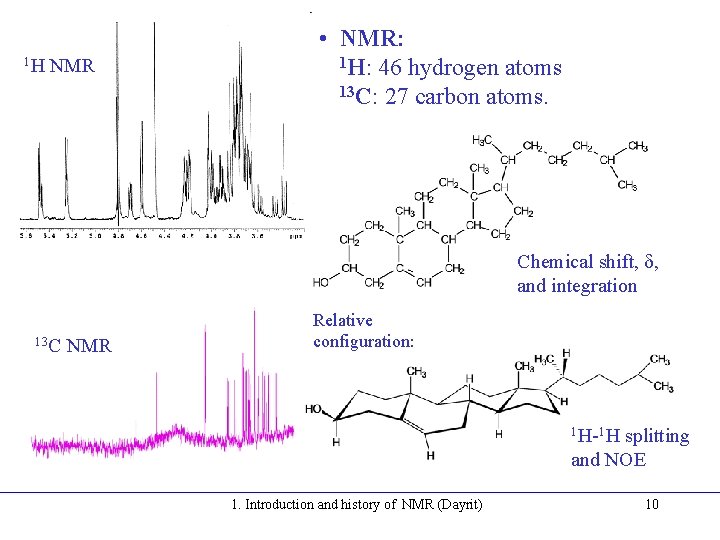
1 H NMR • NMR: 1 H: 46 hydrogen atoms 13 C: 27 carbon atoms. Chemical shift, d, and integration 13 C NMR Relative configuration: 1 H-1 H splitting and NOE 1. Introduction and history of NMR (Dayrit) 10

How does NMR compare with the other spectroscopic methods? • Highest information content Highest complexity • Theory • Sensitivity vs. Resolution • NMR vs. X-ray crystallography • Diversity of application • High cost of technology 1. Introduction and history of NMR (Dayrit) 11

The History of NMR can be divided into the following distinct periods: • 1921 - 45: theories on atomic nuclei (Physics) • 1946 - 55: first NMR experiments (Physics) • 1956 - 65: early structural analysis (Organic Chemistry) • 1966 - 75: first quantum leap in technology and methodology (Chemistry & Electronics) • 1976 - 85: the rise of biological NMR (Biochemistry) • 1986 - present: second quantum leap in technology and methodology (Medicine, Materials Science) 1. Introduction and history of NMR (Dayrit) 12

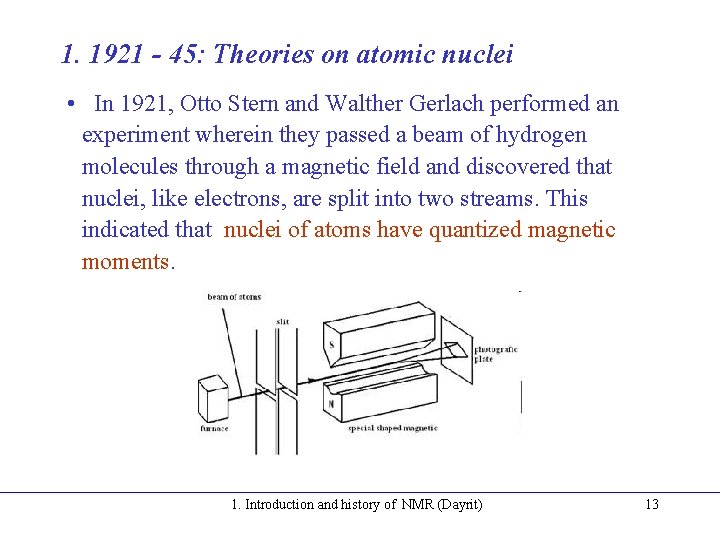
1. 1921 - 45: Theories on atomic nuclei • In 1921, Otto Stern and Walther Gerlach performed an experiment wherein they passed a beam of hydrogen molecules through a magnetic field and discovered that nuclei, like electrons, are split into two streams. This indicated that nuclei of atoms have quantized magnetic moments. 1. Introduction and history of NMR (Dayrit) 13

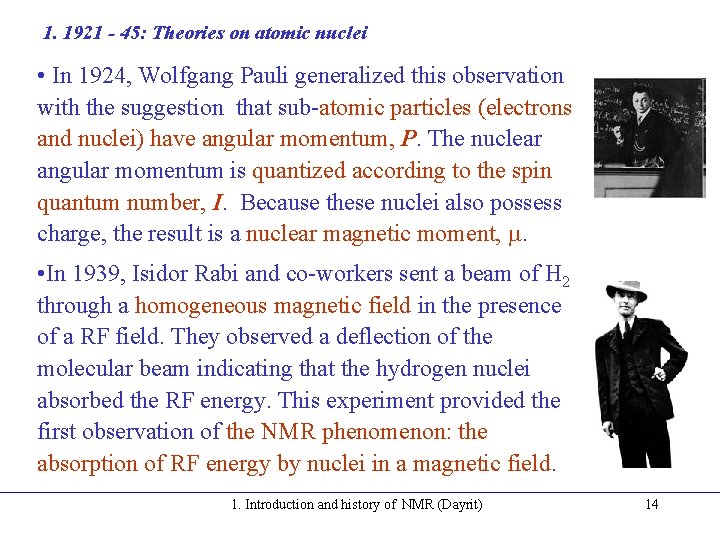
1. 1921 - 45: Theories on atomic nuclei • In 1924, Wolfgang Pauli generalized this observation with the suggestion that sub-atomic particles (electrons and nuclei) have angular momentum, P. The nuclear angular momentum is quantized according to the spin quantum number, I. Because these nuclei also possess charge, the result is a nuclear magnetic moment, . • In 1939, Isidor Rabi and co-workers sent a beam of H 2 through a homogeneous magnetic field in the presence of a RF field. They observed a deflection of the molecular beam indicating that the hydrogen nuclei absorbed the RF energy. This experiment provided the first observation of the NMR phenomenon: the absorption of RF energy by nuclei in a magnetic field. 1. Introduction and history of NMR (Dayrit) 14

1. 1921 - 45: Theories on atomic nuclei • Stern received the Nobel prize in Physics in 1943 his discovery of the magnetic moment of the proton and for the development of the molecular beam method • Rabi received the Nobel prize in Physics in 1944 for developing the resonance method of recording the magnetic properties of atomic nuclei. 1. Introduction and history of NMR (Dayrit) 15

2. 1946 - 55: Early NMR experiments • Early NMR experimental work was done by physicists. In 1946, two groups of physicists, one led by Felix Bloch at Stanford University and the other by Edward Purcell at Harvard University, rushed to apply these theories to the detection of the proton magnetic absorption signal in substances in the liquid or solid phase. Both groups succeeded within a few weeks of each other and simultaneously published their results in the same issue of Physical Reviews, vol. 69 (1946). Felix Bloch Edward Purcell 1. Introduction and history of NMR (Dayrit) 16

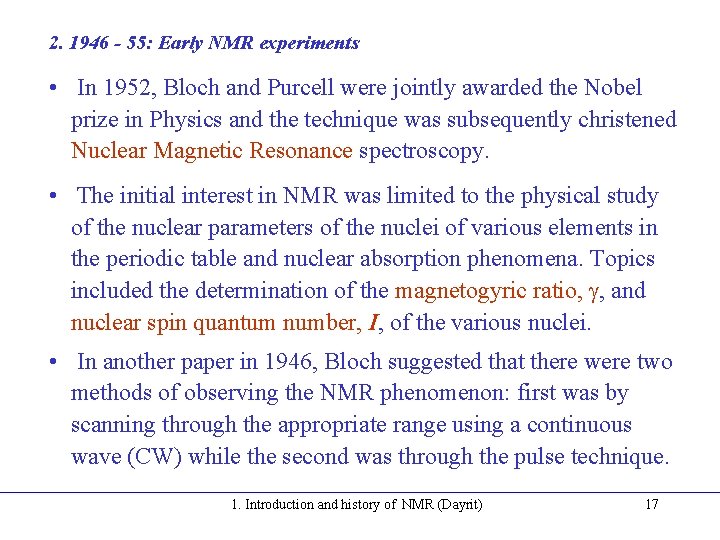
2. 1946 - 55: Early NMR experiments • In 1952, Bloch and Purcell were jointly awarded the Nobel prize in Physics and the technique was subsequently christened Nuclear Magnetic Resonance spectroscopy. • The initial interest in NMR was limited to the physical study of the nuclear parameters of the nuclei of various elements in the periodic table and nuclear absorption phenomena. Topics included the determination of the magnetogyric ratio, , and nuclear spin quantum number, I, of the various nuclei. • In another paper in 1946, Bloch suggested that there were two methods of observing the NMR phenomenon: first was by scanning through the appropriate range using a continuous wave (CW) while the second was through the pulse technique. 1. Introduction and history of NMR (Dayrit) 17

This new property aroused intense interest among physicists because it seemed to offer new insight into nuclear structure. Then (horror of horrors) evidence began to emerge that these fundamental "constants" were not really constant at all, but appeared to depend on the nature of the sample used for the measurement. This disturbing observation is vividly illustrated by the case of ammonium nitrate, investigated by Proctor and Yu in an attempt to derive an accurate value for the magnetic moment of 14 N. To their consternation they observed two resonance lines. Owing to an understandable reluctance to accept that this might be a mere chemical effect, every effort was made to seek a less disturbing explanation. To this end they placed an order for a sample of 15 N-enriched ammonium nitrate in the (vain) hope that the unwanted second resonance line was actually from the 15 N isotope… About the same time, Dickinson found similar "discrepancies" in the 19 F resonance frequencies of several fluorine compounds… and Thomas observed further instances where the proton resonance frequencies depended on the sample used. Something was seriously wrong. How could all the excitement about a fundamental, highprecision nuclear property of such intrinsic importance be so cruelly dashed by a mere chemical shift? Most physicists gave up in disgust. Freeman, 2012

Many setbacks in science turn out to have a silver lining. A few far-sighted scientists began to wonder if this new magnetic resonance discovery could be applied to chemistry, by concentrating attention on the chemical effects so despised by the physicists. Among them, the worldfamous chemist Linus Pauling suggested to Rex Richards at Oxford that NMR might be a promising new avenue of research in chemistry. The justification for such optimism was rather thin at that time, but at least NMR was an open field, and Rex is a courageous researcher. However in that era, a typical chemistry laboratory would be mainly devoted to "wet chemistry" and it was really no place for two-ton magnets. To introduce any large physical machine into that environment was a real battle, involving banishment to some dreary basement room that no one else wanted to use. On the positive side for an NMR pioneer there was a good supply of war-surplus radar equipment that could be usefully cannibalized, and it was always possible to ask those now disillusioned physicists for technical advice. Freeman, 2012

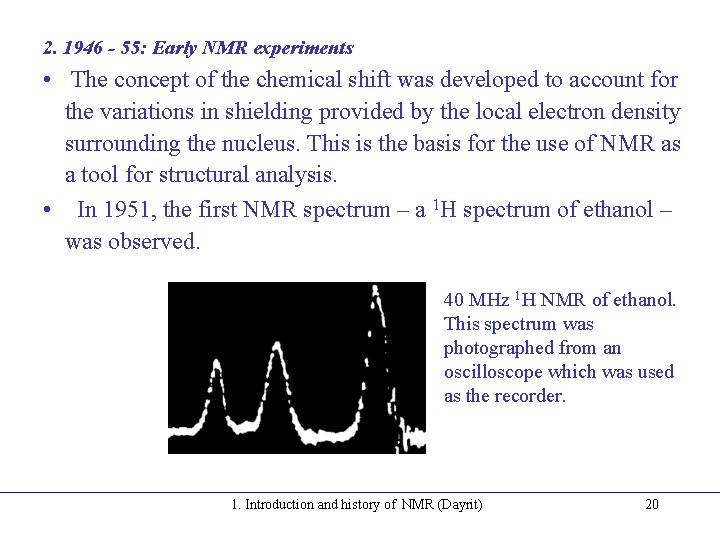
2. 1946 - 55: Early NMR experiments • The concept of the chemical shift was developed to account for the variations in shielding provided by the local electron density surrounding the nucleus. This is the basis for the use of NMR as a tool for structural analysis. • In 1951, the first NMR spectrum – a 1 H spectrum of ethanol – was observed. 40 MHz 1 H NMR of ethanol. This spectrum was photographed from an oscilloscope which was used as the recorder. 1. Introduction and history of NMR (Dayrit) 20

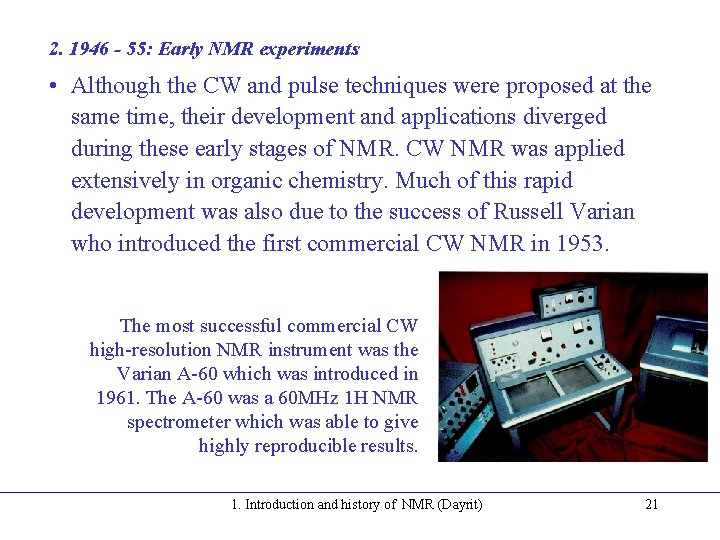
2. 1946 - 55: Early NMR experiments • Although the CW and pulse techniques were proposed at the same time, their development and applications diverged during these early stages of NMR. CW NMR was applied extensively in organic chemistry. Much of this rapid development was also due to the success of Russell Varian who introduced the first commercial CW NMR in 1953. The most successful commercial CW high-resolution NMR instrument was the Varian A-60 which was introduced in 1961. The A-60 was a 60 MHz 1 H NMR spectrometer which was able to give highly reproducible results. 1. Introduction and history of NMR (Dayrit) 21

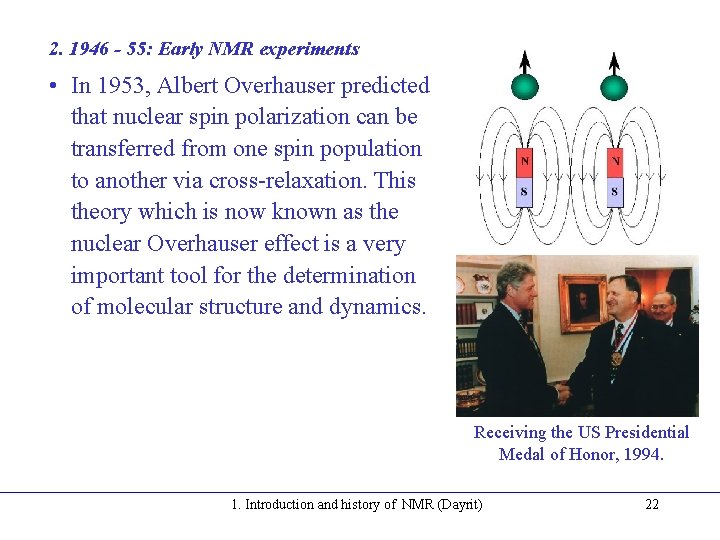
2. 1946 - 55: Early NMR experiments • In 1953, Albert Overhauser predicted that nuclear spin polarization can be transferred from one spin population to another via cross-relaxation. This theory which is now known as the nuclear Overhauser effect is a very important tool for the determination of molecular structure and dynamics. Receiving the US Presidential Medal of Honor, 1994. 1. Introduction and history of NMR (Dayrit) 22

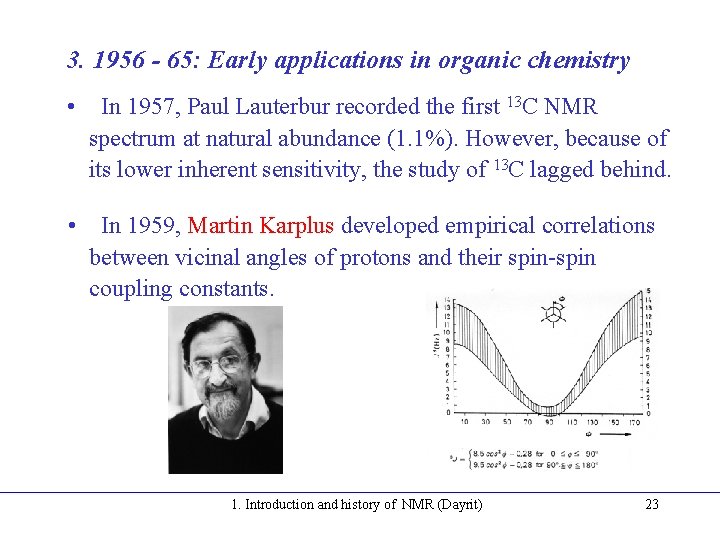
3. 1956 - 65: Early applications in organic chemistry • In 1957, Paul Lauterbur recorded the first 13 C NMR spectrum at natural abundance (1. 1%). However, because of its lower inherent sensitivity, the study of 13 C lagged behind. • In 1959, Martin Karplus developed empirical correlations between vicinal angles of protons and their spin-spin coupling constants. 1. Introduction and history of NMR (Dayrit) 23

4. 1966 - 75: First quantum leap in NMR methodology The main NMR breakthroughs of this decade can be summarized in the following: 1. Application of Fourier transform (FT) to pulse NMR; 2. Development of commercial high-field superconducting magnets; 3. Advances in electronics and computers; and 4. These developments in turn made possible the development of 2 -D NMR. 1. Introduction and history of NMR (Dayrit) 24

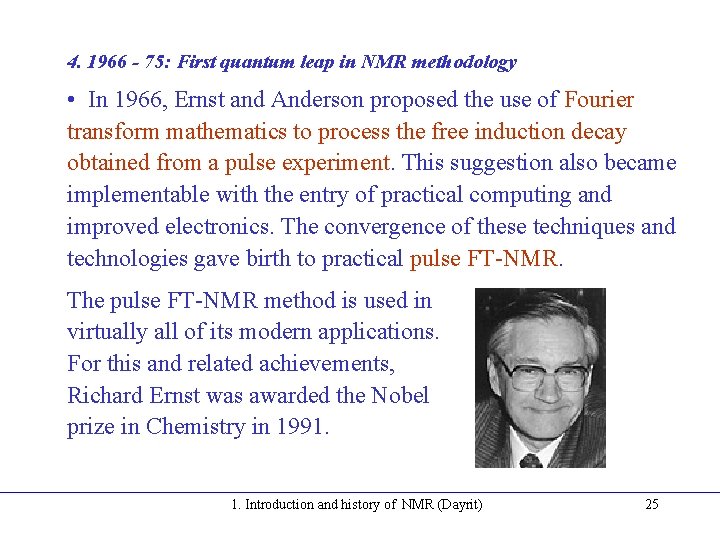
4. 1966 - 75: First quantum leap in NMR methodology • In 1966, Ernst and Anderson proposed the use of Fourier transform mathematics to process the free induction decay obtained from a pulse experiment. This suggestion also became implementable with the entry of practical computing and improved electronics. The convergence of these techniques and technologies gave birth to practical pulse FT-NMR. The pulse FT-NMR method is used in virtually all of its modern applications. For this and related achievements, Richard Ernst was awarded the Nobel prize in Chemistry in 1991. 1. Introduction and history of NMR (Dayrit) 25

4. 1966 - 75: First quantum leap in NMR methodology • The workhorse NMR equipment were based on the iron magnet. Unfortunately, the upper limit of iron magnets is about 2. 35 tesla (100 -MHz 1 H NMR). The development of superconducting magnets enabled the development of magnetic fields in commercial instruments of 6. 35 T, 8. 46 T and 9. 40 T (270, 360, 400 MHz). • The implementation of pulse FT-NMR was made possible by the rapid rise in computer and electronics technology because such techniques require the rapid and accurate accumulation of data and their processing. In addition, experiments requiring many hours of accumulation became possible with improved electronics and computer technology. 1. Introduction and history of NMR (Dayrit) 26

4. 1966 - 75: First quantum leap in NMR methodology • In 1971, Jean Jeener suggested the use of two independent time domains as a means of obtaining new NMR information. This resulted in the birth of 2 -dimensional NMR. 2 -D NMR revolutionized the practice of NMR. • In 1978, N. Watanabe at JEOL reported the coupling of a liquid chromatograph to an NMR using a stopped flow technique. An on-line system was developed a year later. . This was the first report of a “hyphenated” NMR technique. 1. Introduction and history of NMR (Dayrit) 27

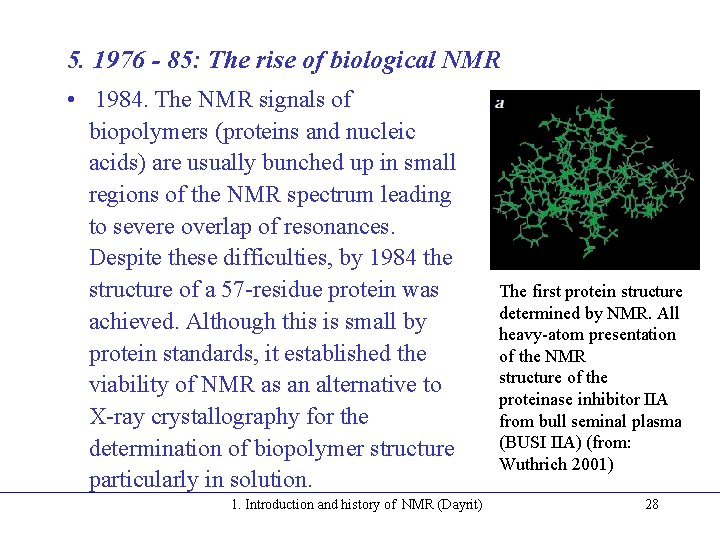
5. 1976 - 85: The rise of biological NMR • 1984. The NMR signals of biopolymers (proteins and nucleic acids) are usually bunched up in small regions of the NMR spectrum leading to severe overlap of resonances. Despite these difficulties, by 1984 the structure of a 57 -residue protein was achieved. Although this is small by protein standards, it established the viability of NMR as an alternative to X-ray crystallography for the determination of biopolymer structure particularly in solution. 1. Introduction and history of NMR (Dayrit) The first protein structure determined by NMR. All heavy-atom presentation of the NMR structure of the proteinase inhibitor IIA from bull seminal plasma (BUSI IIA) (from: Wuthrich 2001) 28

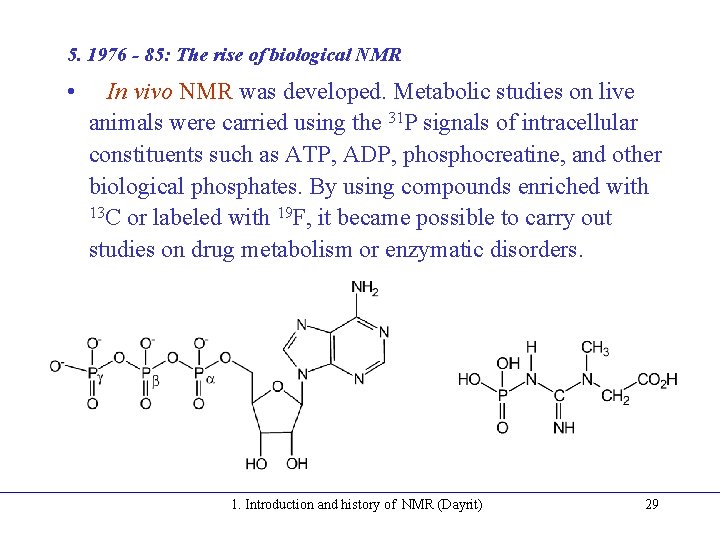
5. 1976 - 85: The rise of biological NMR • In vivo NMR was developed. Metabolic studies on live animals were carried using the 31 P signals of intracellular constituents such as ATP, ADP, phosphocreatine, and other biological phosphates. By using compounds enriched with 13 C or labeled with 19 F, it became possible to carry out studies on drug metabolism or enzymatic disorders. 1. Introduction and history of NMR (Dayrit) 29

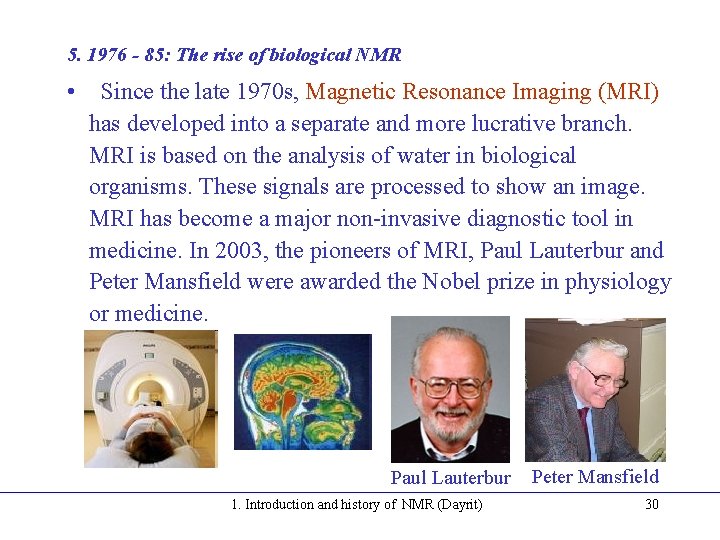
5. 1976 - 85: The rise of biological NMR • Since the late 1970 s, Magnetic Resonance Imaging (MRI) has developed into a separate and more lucrative branch. MRI is based on the analysis of water in biological organisms. These signals are processed to show an image. MRI has become a major non-invasive diagnostic tool in medicine. In 2003, the pioneers of MRI, Paul Lauterbur and Peter Mansfield were awarded the Nobel prize in physiology or medicine. Paul Lauterbur 1. Introduction and history of NMR (Dayrit) Peter Mansfield 30

6. 1986 - present: second quantum in technology and methodology • The rapid increase in computing capabilities and magnetic field strengths (now up to 1 GHz) has extended the sizes of proteins that can be studied. • NMR has also been applied to the field of materials science following developments in solid state NMR; the availability of broad band transmitters means that virtually any element in the periodic table can be studied. 1. Introduction and history of NMR (Dayrit) 31

6. 1986 - present: second quantum in technology and methodology • The use of NMR in routine analytical chemistry is expanding. NMR methods have been developed for analysis in the food, fats and oils, paint, and polymer industries. For example, it is used to determine moisture, fat, and solid content of products, ranging from chocolate, seed, vegetable oil, plant, among others. • Although NMR has been largely used as a qualitative technique, the quantitative applications of NMR are now being developed. Low-resolution NMR for analysis of solid fat content. 1. Introduction and history of NMR (Dayrit) 32

6. 1986 - present: second quantum in technology and methodology • NMR is one of the principal methods, along with Mass Spectrometry, in the field of Proteomics. The RIKEN “NMR Park” Yokohama, Japan. 1. Introduction and history of NMR (Dayrit) 33

7. Current applications in NMR • Organic structure determination • Inorganic systems: metal or ligand analysis (e. g. , 31 P, 15 N, 13 C, 17 O) • Medical applications for diagnostics: • functional MRI (f. MRI) • Image processing • Structural Biology • Proteins, protein dynamics protein-drug interaction, membrane proteins • Metalloproteins 1. Introduction and history of NMR (Dayrit) 34

7. Current applications in NMR • Materials Science • Solids NMR • Sensitivity for multi-nuclear detection • High temperature, high pressure cells • Polymers: • Polymer structure • Natural polymers • Metabolomics • Small molecules in urine • Quantitative NMR • Industrial applications • NMR vs. X-ray 1. Introduction and history of NMR (Dayrit) 35