Ch 23 Acids Bases I Intro to Acids

Ch. 23 - Acids & Bases I. Intro to Acids & Bases u u u Definitions Properties Uses
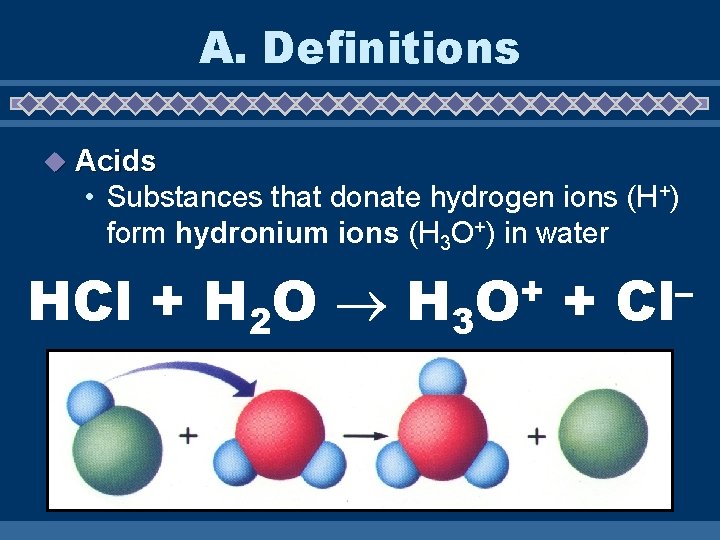
A. Definitions u Acids • Substances that donate hydrogen ions (H+) form hydronium ions (H 3 O+) in water HCl + H 2 O H 3 + O + – Cl

Properties of Acids u sour taste u corrosive u electrolytes u turn litmus red u react with metals to form H 2 gas u Strength of acid depends on how well they form H+ in water. u p. H less than 7
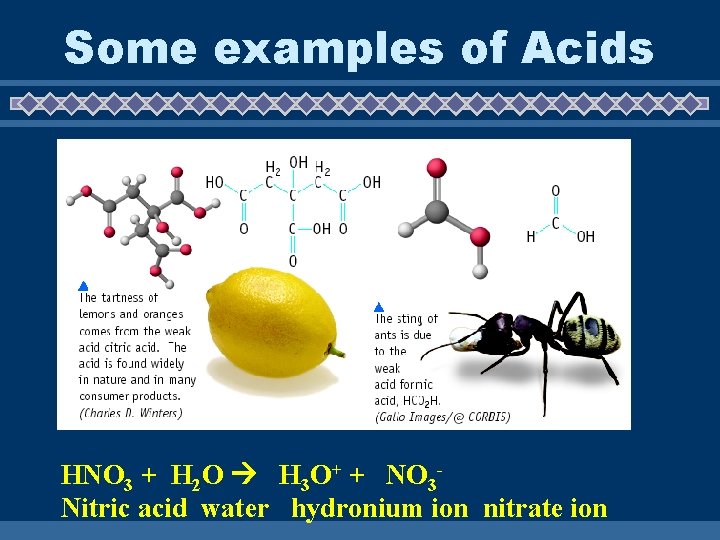
Some examples of Acids HNO 3 + H 2 O H 3 O+ + NO 3 Nitric acid water hydronium ion nitrate ion

Some Examples of Acids

Digesting Your Food Your stomach contains two strong acids: HCl (hydrochloric acid and pepsin).

More examples of Acids u H 3 PO 4 - soft drinks, fertilizer, detergents u H 2 SO 4 - fertilizer, car batteries u HCl - gastric juice u HC 2 H 3 O 2 - vinegar
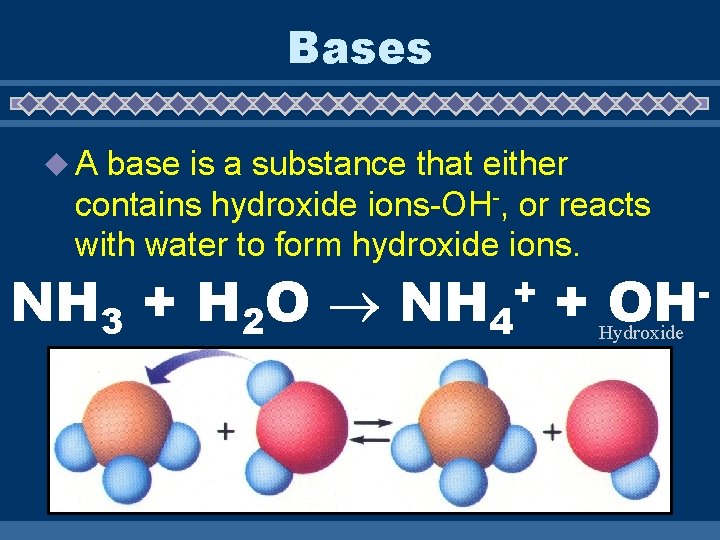
Bases u. A base is a substance that either contains hydroxide ions-OH-, or reacts with water to form hydroxide ions. + NH 3 + H 2 O NH 4 + Hydroxide OH

Properties of Bases u bitter taste u corrosive u electrolytes u turn litmus blue u slippery u p. H feel greater than 7

Some examples of Bases • KOH K+ + OH • potassium hydroxide -> potassium ion plus hydroxide Many soaps contain bases!

Some Common Bases & Uses u Na. OH - lye, drain and oven cleaner, hair relaxers u Mg(OH)2 - laxative, antacid- “MOM” Milk of magnesia u NH 3 - cleaners, fertilizer l Al(OH)3 aluminum hydroxide Maalox (antacid)

Strengths of Acids and Bases u Strong Acid/Base • 100% ions in water • strong electrolyte • HCl, HNO 3, Na. OH, Li. OH u Weak Acid/Base • few ions in water • weak electrolyte • HC 2 H 3 O 2, NH 3 - +
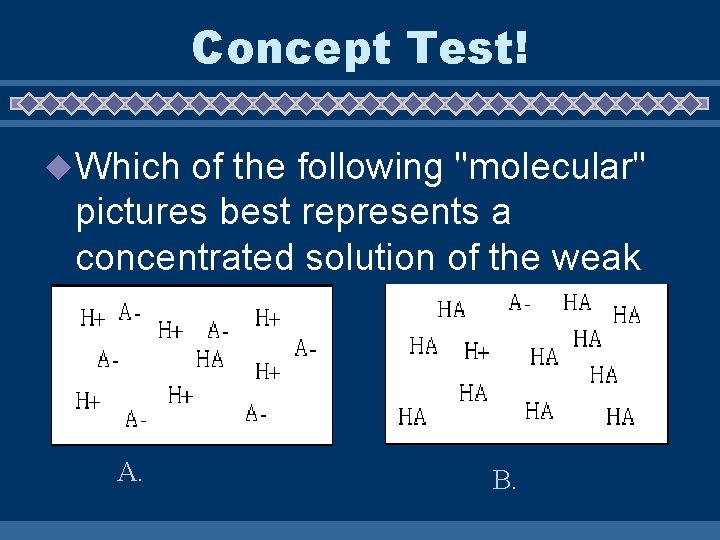
Concept Test! u Which of the following "molecular" pictures best represents a concentrated solution of the weak acid HA? A. B.
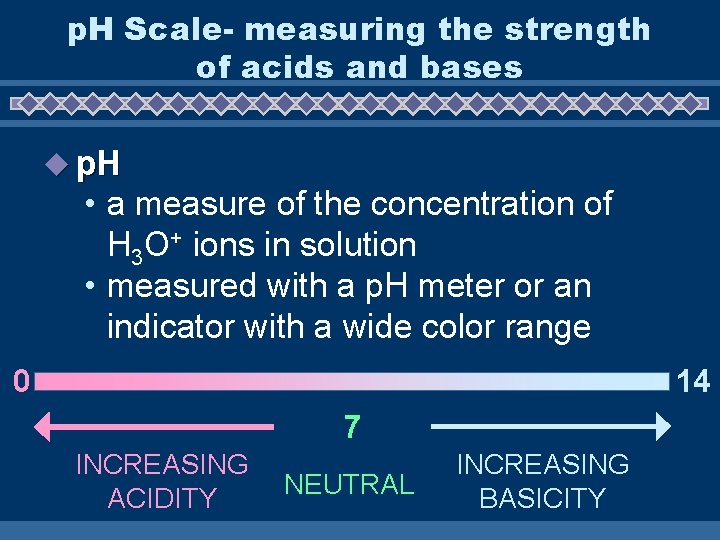
p. H Scale- measuring the strength of acids and bases u p. H • a measure of the concentration of H 3 O+ ions in solution • measured with a p. H meter or an indicator with a wide color range 14 0 7 INCREASING ACIDITY NEUTRAL INCREASING BASICITY
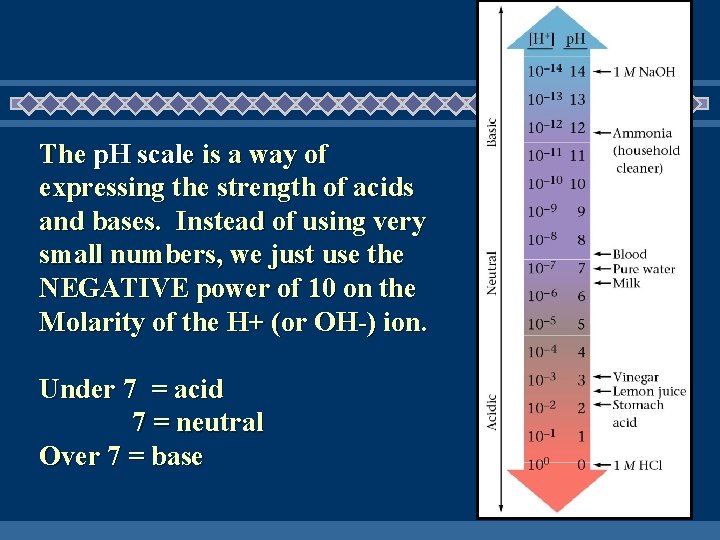
The p. H scale is a way of expressing the strength of acids and bases. Instead of using very small numbers, we just use the NEGATIVE power of 10 on the Molarity of the H+ (or OH-) ion. Under 7 = acid 7 = neutral Over 7 = base
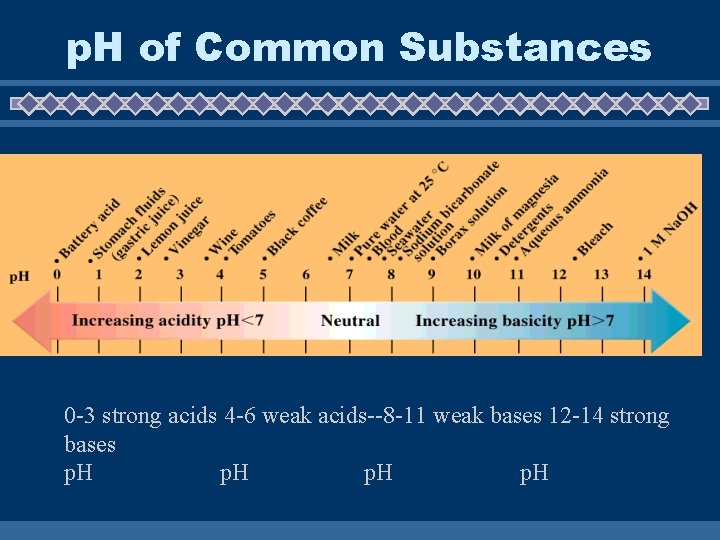
p. H of Common Substances 0 -3 strong acids 4 -6 weak acids--8 -11 weak bases 12 -14 strong bases p. H

p. H Testing u Indicator • substance that changes color in an acid or base u Examples: • Litmus paper - red/blue • phenolphthalein - colorless/pink • goldenrod - yellow/red • red cabbage juice (natural indicator)pink/green

Neutralization Reactions u u u u When an acid and a base react, they produce a salt and water. Acid + Base salt + water HCl + Na. OH Na. Cl + H 2 O The p. H of the products will be nearly neutral or 7. 0. Neutralization to the rescue! When a strong acid is spilled in the lab, it can be neutralized using a base – like baking soda. When a strong base is spilled, it can be neutralized using an acid-like vinegar. Both will produce salt-water with a p. H of near 7. 0.
A common neutralization reaction! People use antacids such as Tums or Rolaids to neutralize stomach acid (HCl) from overeating and drinking. u Antacids are weak bases. Baking soda and water can also be used. u What do these weak antacids do to the excess stomach acid? u
- Slides: 19