CH 22 HYDROCARBON COMPOUNDS 22 1 HYDROCARBONS Organic

CH. 22 HYDROCARBON COMPOUNDS

22. 1 HYDROCARBONS � Organic Chemistry � includes almost all carbon compounds � Not limited to compounds found in living cells � Hydrocarbon: organic compound made of only hydrogen and carbon atoms
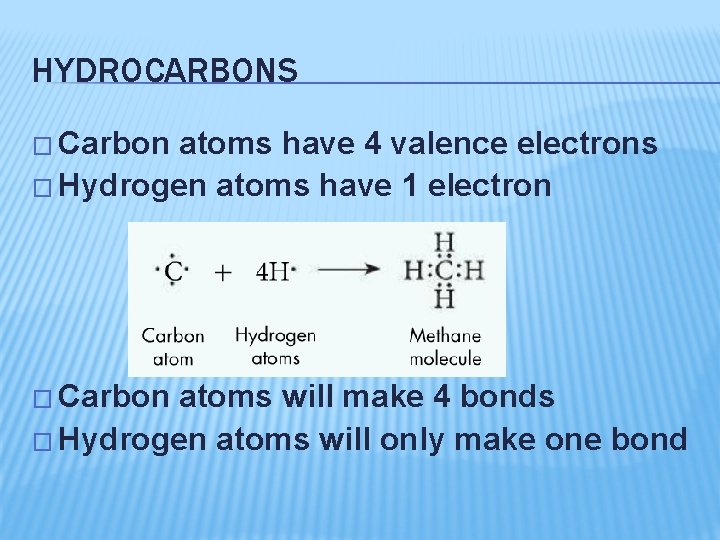
HYDROCARBONS � Carbon atoms have 4 valence electrons � Hydrogen atoms have 1 electron � Carbon atoms will make 4 bonds � Hydrogen atoms will only make one bond
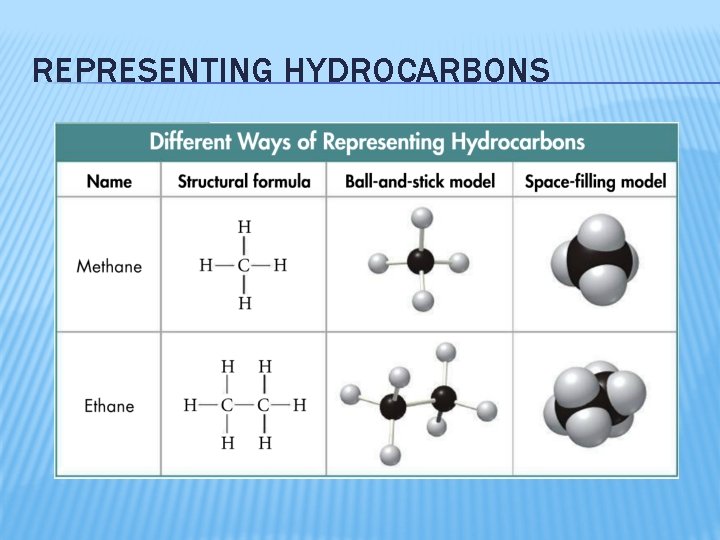
REPRESENTING HYDROCARBONS

PROPERTIES OF HYDROCARBONS � Nonpolar molecules � Will not mix with water � Mix with other nonpolar molecules � Tend to be gas or liquid (with low BP) at room temperature � Combustible in presence of oxygen and heat
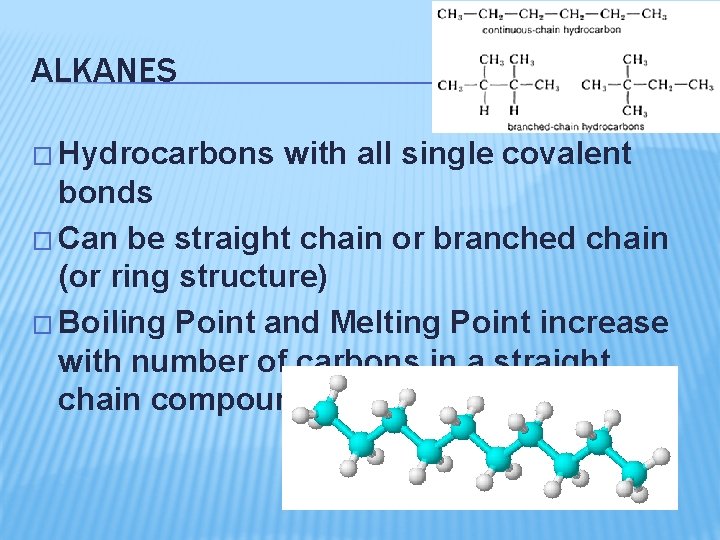
ALKANES � Hydrocarbons with all single covalent bonds � Can be straight chain or branched chain (or ring structure) � Boiling Point and Melting Point increase with number of carbons in a straight chain compound

NAMING STRAIGHT CHAIN ALKANES � IUPAC: International Union of Pure and Applied Chemistry � Name ends with suffix – ane � Prefix indicates the number of carbon atoms � Note: the prefixes for the first 4 carbons are different than the prefixes
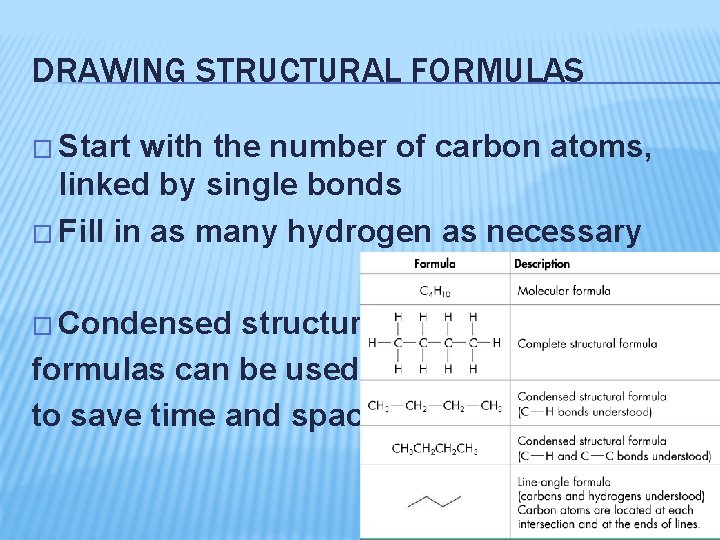
DRAWING STRUCTURAL FORMULAS � Start with the number of carbon atoms, linked by single bonds � Fill in as many hydrogen as necessary � Condensed structural formulas can be used to save time and space

PRACTICE � Draw complete structural formulas for straight chain alkanes with a) Three carbon atoms b) Four carbon atoms c) Seven carbon atoms � Name each of them.

PRACTICE � Draw a) b) c) the complete structural formula for Ethane Nonane Methane

PRACTICE � How many single bonds are there in a propane molecule? � How many single bonds are there in a butane molecule?

BRANCHED-CHAIN HYDROCARBONS � One carbon atom is bonded to 3 or 4 other carbon atoms � Branches are named as substituent groups � The longest continuous carbon chain is the parent alkane (provides number of carbons for the name prefix) � Hydrocarbon substituents are called alkyl groups � Methyl � Ethyl � Propyl � Butyl � Pentyl, etc.
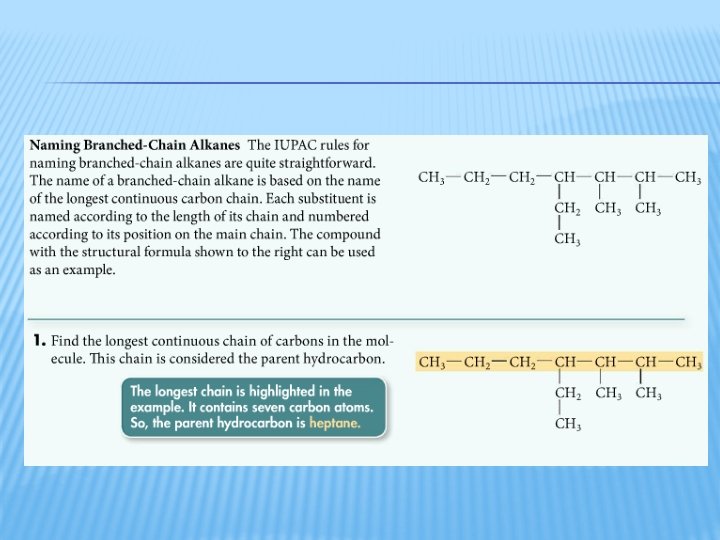


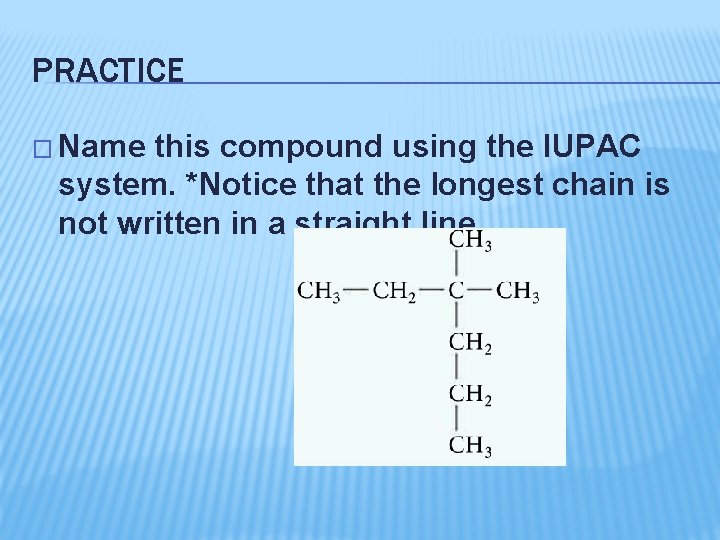
PRACTICE � Name this compound using the IUPAC system. *Notice that the longest chain is not written in a straight line.
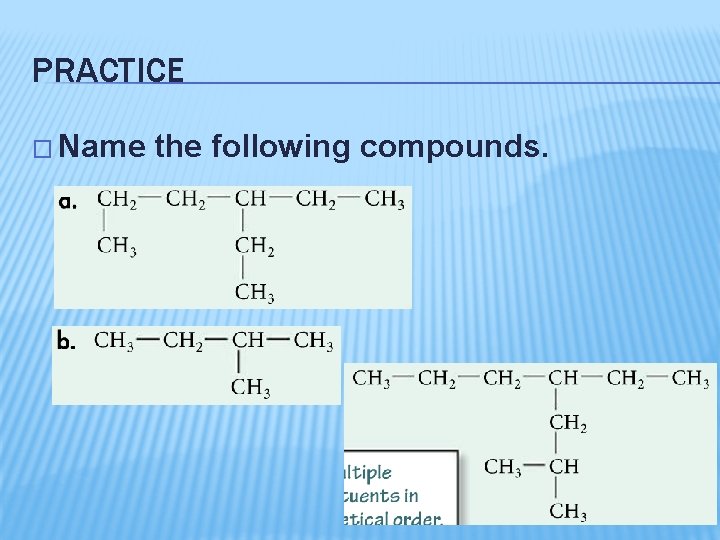
PRACTICE � Name the following compounds.

PRACTICE � Draw the structural formula for 3 -ethyl-2, 4 -dimethylhexane. � Draw the structural formula for 2, 3 dimethylbutane. � Draw the structural formula for 3, 4 -diethyl -2, 5 -dimethyloctane.
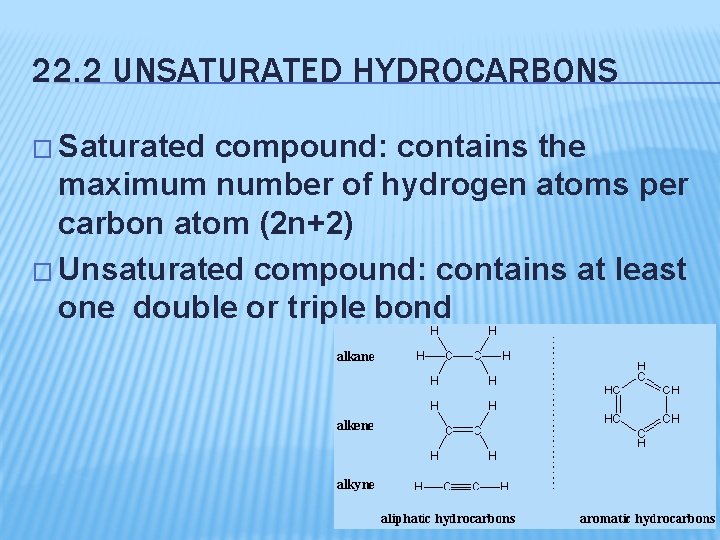
22. 2 UNSATURATED HYDROCARBONS � Saturated compound: contains the maximum number of hydrogen atoms per carbon atom (2 n+2) � Unsaturated compound: contains at least one double or triple bond
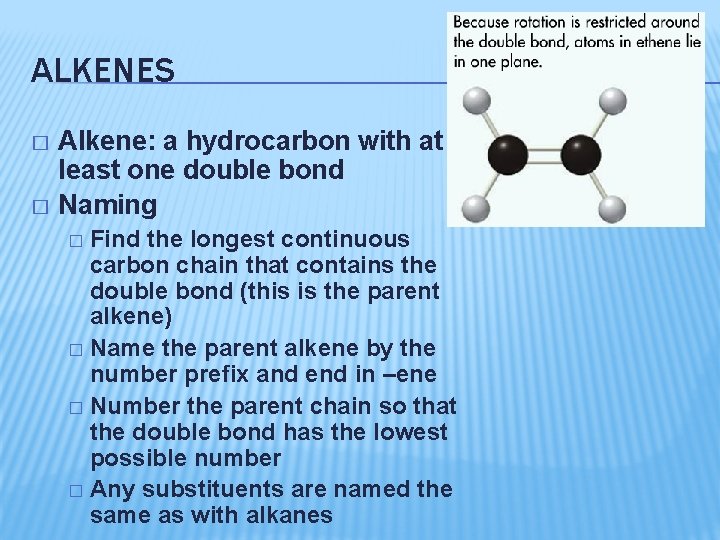
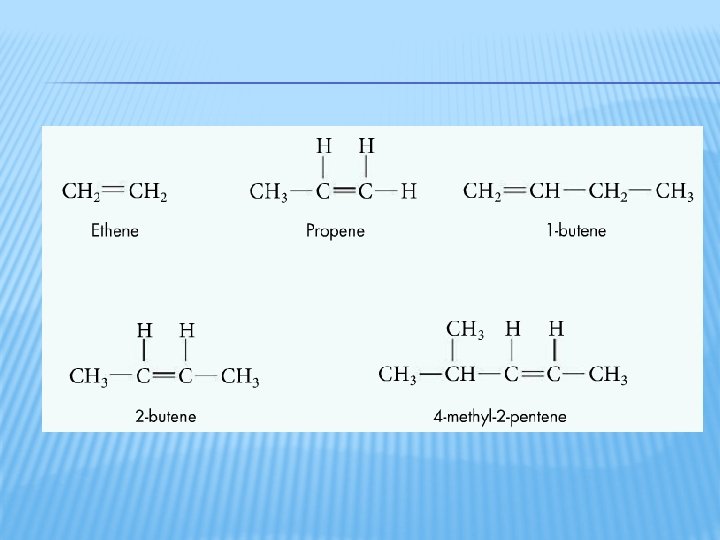
ALKENES Alkene: a hydrocarbon with at least one double bond � Naming � Find the longest continuous carbon chain that contains the double bond (this is the parent alkene) � Name the parent alkene by the number prefix and end in –ene � Number the parent chain so that the double bond has the lowest possible number � Any substituents are named the same as with alkanes �
ALKYNES � Alkyne: a hydrocarbon with at least one triple bond � Naming � Number the parent chain so that the triple bond has the lowest possible number on the longest continuous chain � Name the parent chain with the number prefix � End the name in –yne � Name substituents as with alkanes

PRACTICE � Draw a complete structural formula for each of the following hydrocarbon compounds. �Ethane �Ethene �Ethyne �Propane �Propene �Propyne
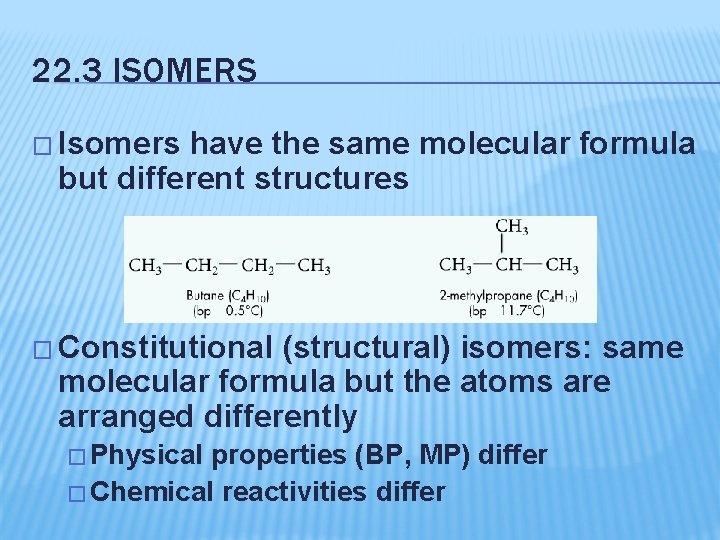
22. 3 ISOMERS � Isomers have the same molecular formula but different structures � Constitutional (structural) isomers: same molecular formula but the atoms are arranged differently � Physical properties (BP, MP) differ � Chemical reactivities differ

STEREOISOMERS � Stereoisomers: atoms are joined in same order, but in different arrangements in space � Cis-trans isomers � Enantiomers
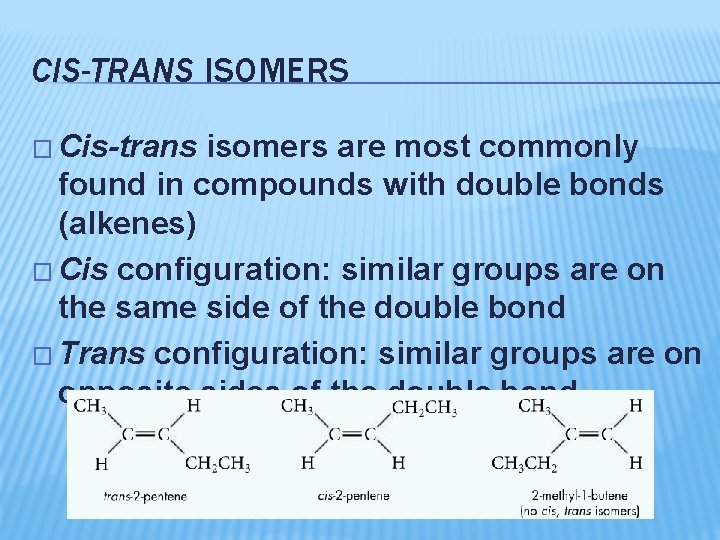
CIS-TRANS ISOMERS � Cis-trans isomers are most commonly found in compounds with double bonds (alkenes) � Cis configuration: similar groups are on the same side of the double bond � Trans configuration: similar groups are on opposite sides of the double bond

ENANTIOMERS � Occurs when a central atom (C) has four different atoms or groups attached � When this central atom is carbon, it is called an asymmetric carbon � Groups attached to an asymmetric carbon can form mirror images of each other � When these mirror images are not superimposable, the two forms are called enantiomers � The enantiomers have identical physical
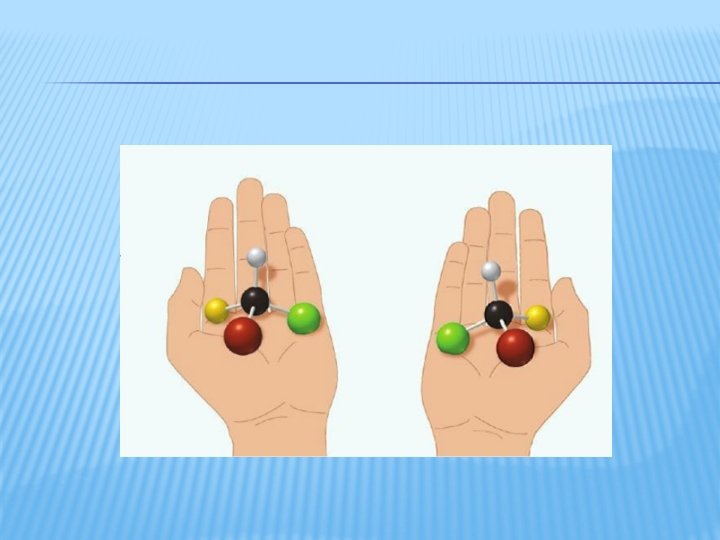

PRACTICE
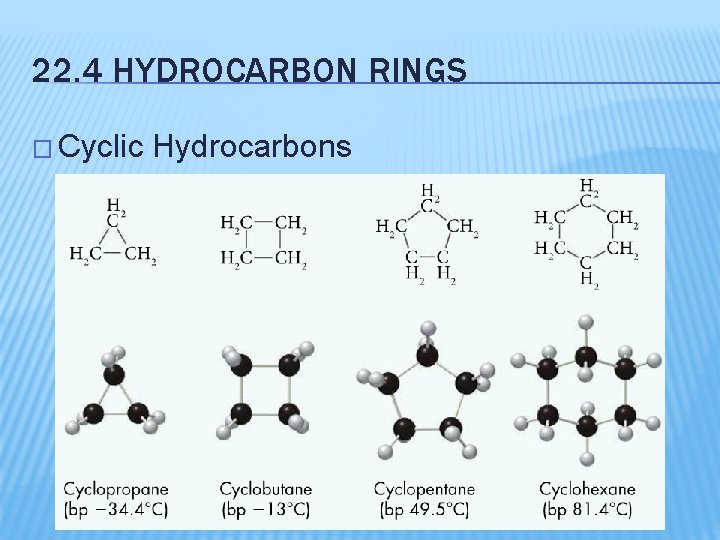
22. 4 HYDROCARBON RINGS � Cyclic Hydrocarbons
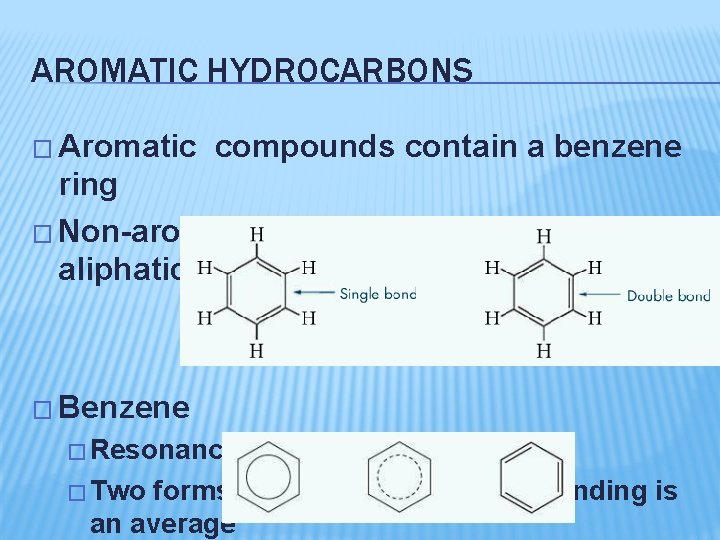
AROMATIC HYDROCARBONS � Aromatic compounds contain a benzene ring � Non-aromatic compounds are called aliphatic � Benzene � Resonance increases stability � Two forms are extremes, the true bonding is an average
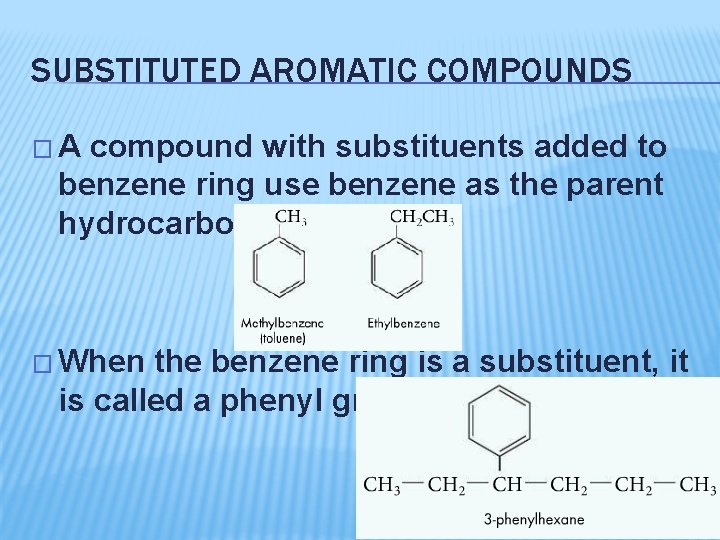
SUBSTITUTED AROMATIC COMPOUNDS �A compound with substituents added to benzene ring use benzene as the parent hydrocarbon � When the benzene ring is a substituent, it is called a phenyl group
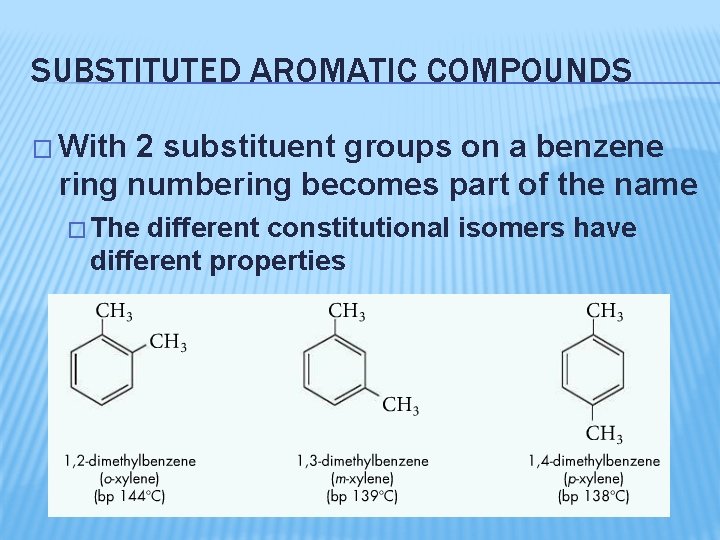
SUBSTITUTED AROMATIC COMPOUNDS � With 2 substituent groups on a benzene ring numbering becomes part of the name � The different constitutional isomers have different properties

22. 5 HYDROCARBONS FROM EARTH’S CRUST � Natural Gas � Source of low-mass alkanes � 80% methane, 10% ethane, 4% propane, 2% butane � 4% contains nitrogen, higher mass hydrocarbons, and helium � Combustion requires O 2 gas � Methane burns with a hot, clean flame

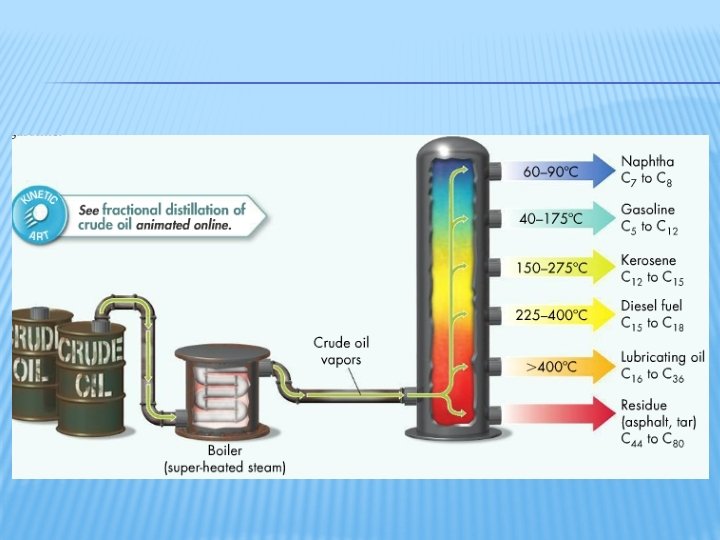
22. 5 HYDROCARBONS FROM EARTH’S CRUST � Petroleum � More complex compounds than in natural gas � Mostly straight or branched-chain alkanes � Includes some aromatic compounds and sulfur-, oxygen-, or nitrogen-containing compounds � Petroleum refining begins with distilling crude oil into fractions by boiling point � Cracking breaks down larger hydrocarbons into smaller, more useful hydrocarbons

BIOREMEDIATION � Using oil-eating microbes (mostly bacteria) to clean up an oil-spill � Microbes digest crude oil into mostly CO 2 and H 2 O � Takes time to work � Most effective along shorelines after other means of cleaning

22. 5 HYDROCARBONS FROM EARTH’S CRUST � Coal � Classified by hardness and carbon content � Mostly condensed aromatic hydrocarbons with high mass � Leaves more soot when burned � Forms in multiple stages from pressure and heat over time � Peat lignite bituminous anthracite
- Slides: 38