Ch 2 Continued Examples Ionic Bonding Predominant bonding
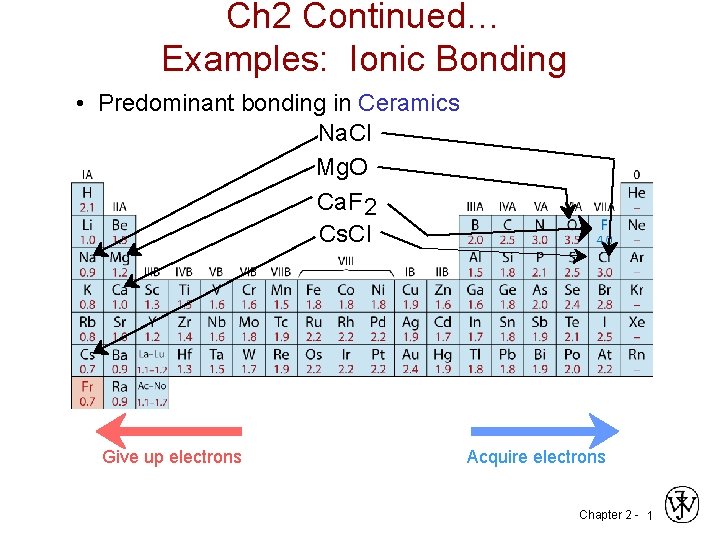
Ch 2 Continued… Examples: Ionic Bonding • Predominant bonding in Ceramics Na. Cl Mg. O Ca. F 2 Cs. Cl Give up electrons Acquire electrons Chapter 2 - 1
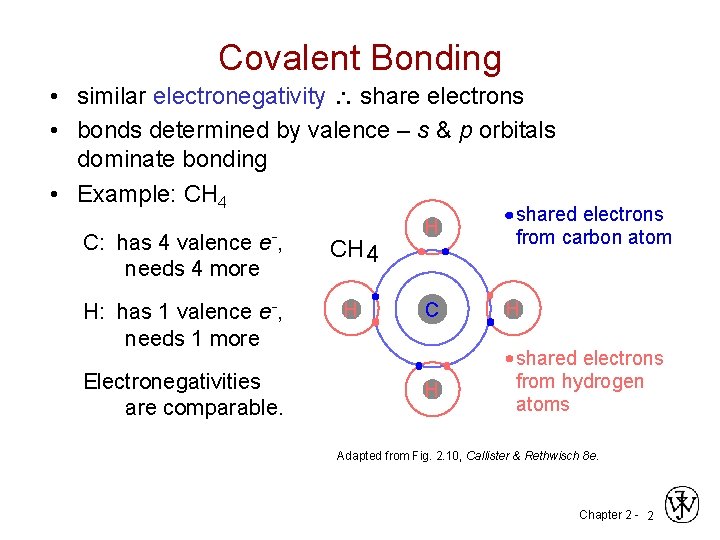
Covalent Bonding • similar electronegativity share electrons • bonds determined by valence – s & p orbitals dominate bonding • Example: CH 4 C: has 4 valence e-, needs 4 more CH 4 H: has 1 valence e-, needs 1 more H Electronegativities are comparable. H C H shared electrons from carbon atom H shared electrons from hydrogen atoms Adapted from Fig. 2. 10, Callister & Rethwisch 8 e. Chapter 2 - 2
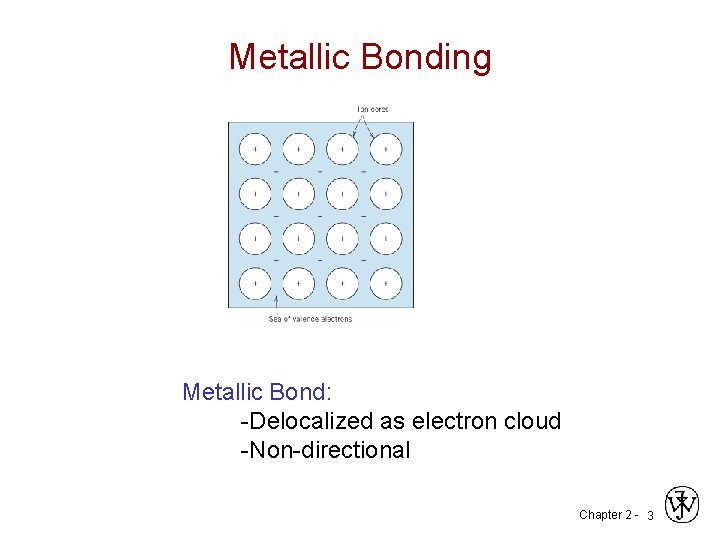
Metallic Bonding Metallic Bond: -Delocalized as electron cloud -Non-directional Chapter 2 - 3

Mixed Bonding • Ionic-Covalent Mixed Bonding % ionic character = x (100%) where XA & XB are Pauling electronegativities Ex: Mg. O XMg = 1. 2 XO = 3. 5 Chapter 2 - 4
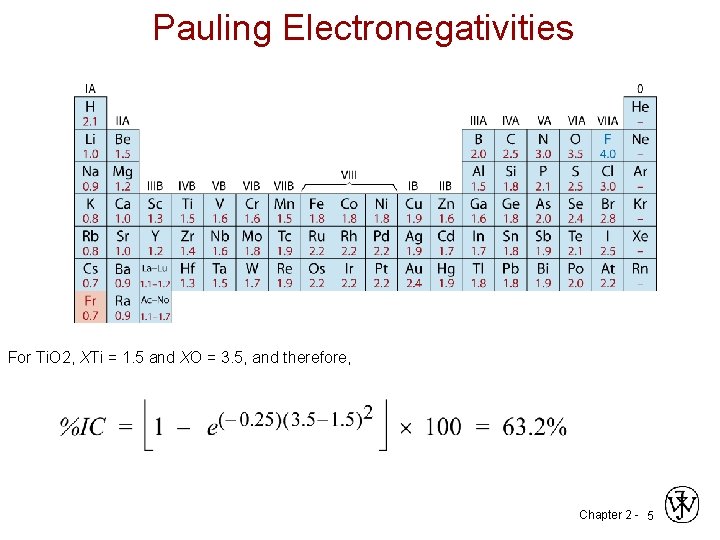
Pauling Electronegativities For Ti. O 2, XTi = 1. 5 and XO = 3. 5, and therefore, Chapter 2 - 5
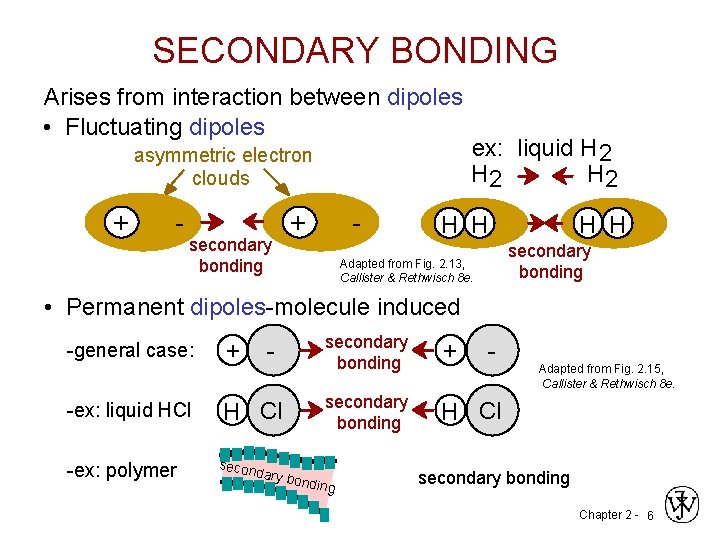
SECONDARY BONDING Arises from interaction between dipoles • Fluctuating dipoles asymmetric electron clouds + - secondary bonding + - ex: liquid H 2 H 2 H H secondary bonding Adapted from Fig. 2. 13, Callister & Rethwisch 8 e. • Permanent dipoles-molecule induced -general case: -ex: liquid HCl -ex: polymer + - H Cl secon dary b secondary bonding + secondary bonding H Cl ondin g - Adapted from Fig. 2. 15, Callister & Rethwisch 8 e. secondary bonding Chapter 2 - 6
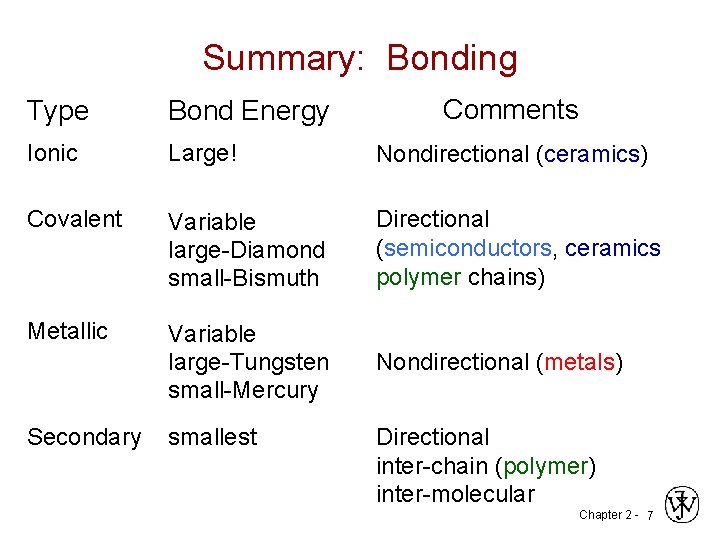
Summary: Bonding Comments Type Bond Energy Ionic Large! Nondirectional (ceramics) Covalent Variable large-Diamond small-Bismuth Directional (semiconductors, ceramics polymer chains) Metallic Variable large-Tungsten small-Mercury Nondirectional (metals) Secondary smallest Directional inter-chain (polymer) inter-molecular Chapter 2 - 7
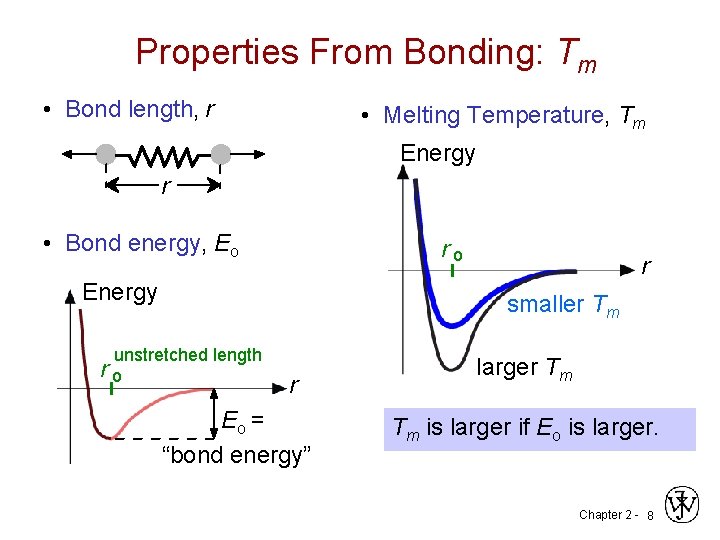
Properties From Bonding: Tm • Bond length, r • Melting Temperature, Tm Energy r • Bond energy, Eo ro Energy r smaller Tm unstretched length ro r Eo = “bond energy” larger Tm Tm is larger if Eo is larger. Chapter 2 - 8
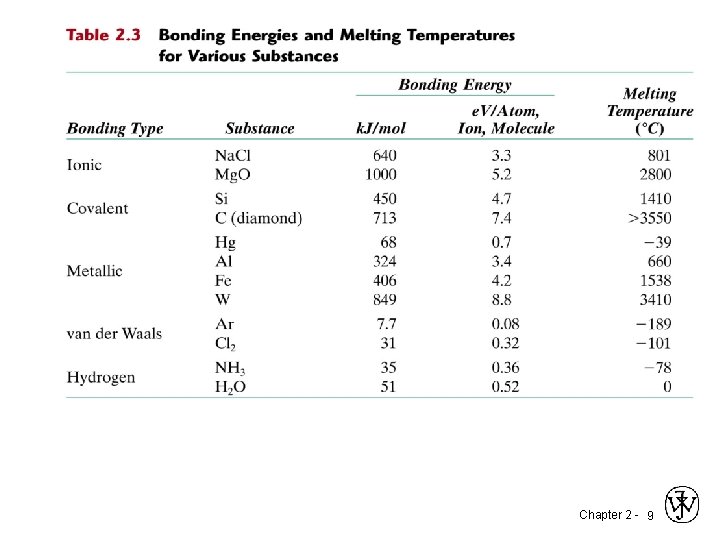
Chapter 2 - 9
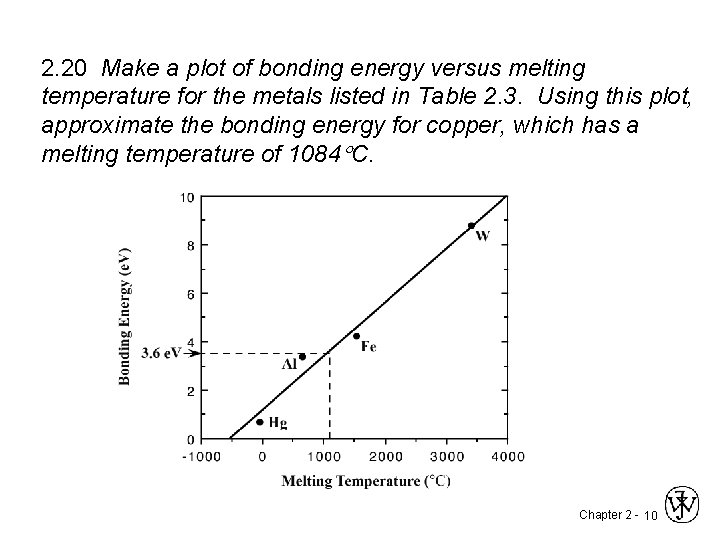
2. 20 Make a plot of bonding energy versus melting temperature for the metals listed in Table 2. 3. Using this plot, approximate the bonding energy for copper, which has a melting temperature of 1084 C. Chapter 2 - 10
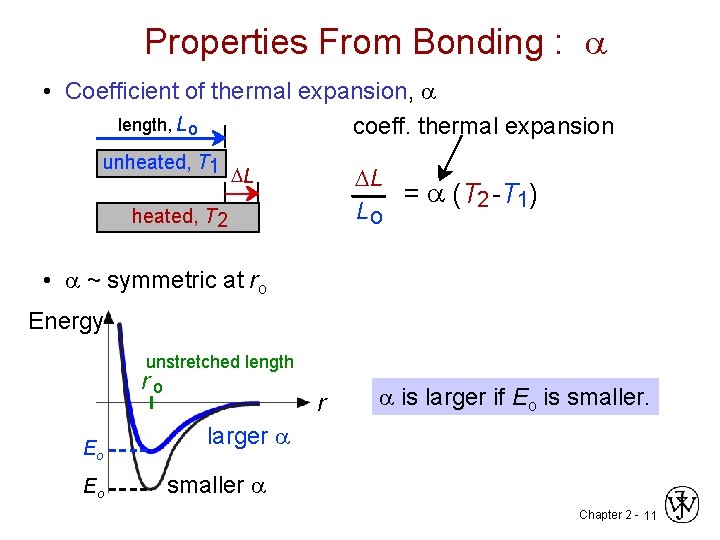
Properties From Bonding : a • Coefficient of thermal expansion, a length, L o coeff. thermal expansion unheated, T 1 DL = a (T 2 -T 1) Lo DL heated, T 2 • a ~ symmetric at ro Energy unstretched length ro Eo Eo r a is larger if Eo is smaller. larger a smaller a Chapter 2 - 11
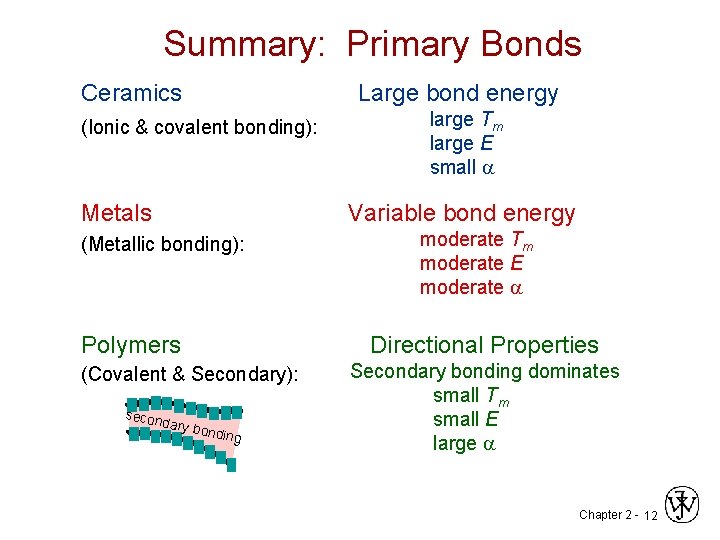
Summary: Primary Bonds Ceramics Large bond energy (Ionic & covalent bonding): Metals large Tm large E small a Variable bond energy (Metallic bonding): Polymers Directional Properties (Covalent & Secondary): secon moderate Tm moderate E moderate a dary b o nding Secondary bonding dominates small Tm small E large a Chapter 2 - 12
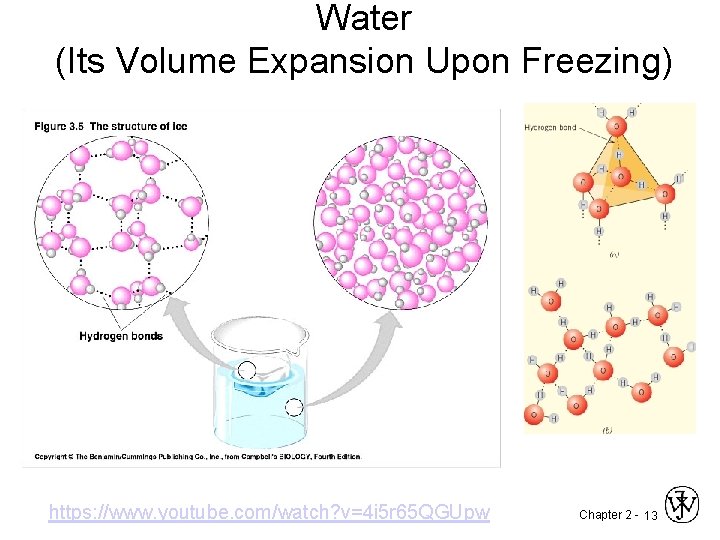
Water (Its Volume Expansion Upon Freezing) https: //www. youtube. com/watch? v=4 i 5 r 65 QGUpw Chapter 2 - 13
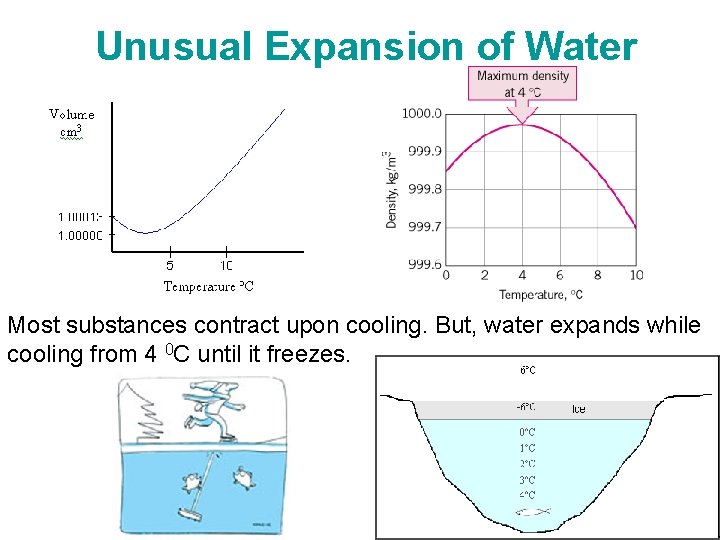
Unusual Expansion of Water Most substances contract upon cooling. But, water expands while cooling from 4 0 C until it freezes. Chapter 2 -
- Slides: 14