Ch 2 Continued Examples Ionic Bonding Na Cl
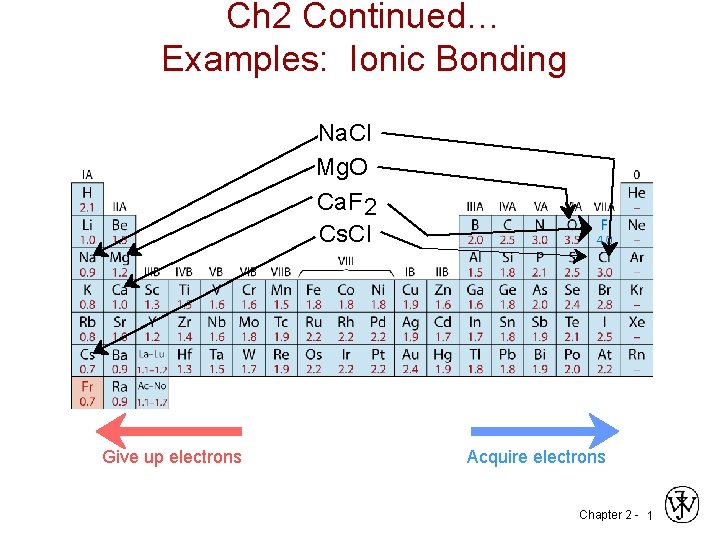
Ch 2 Continued… Examples: Ionic Bonding Na. Cl Mg. O Ca. F 2 Cs. Cl Give up electrons Acquire electrons Chapter 2 - 1
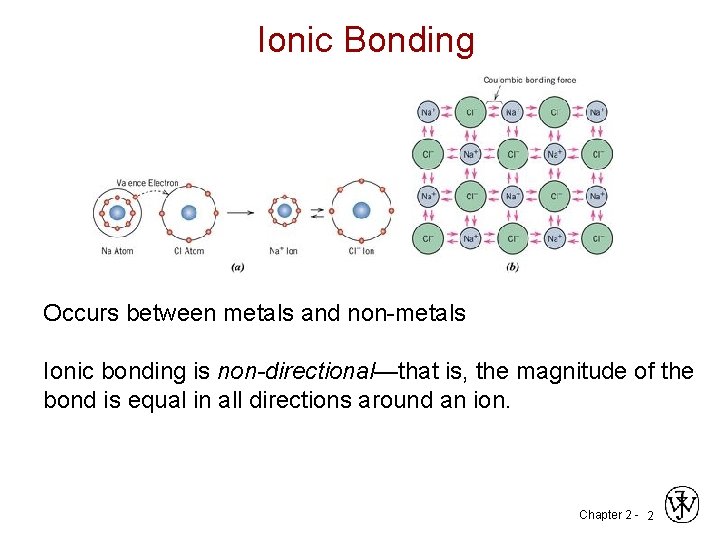
Ionic Bonding Occurs between metals and non-metals Ionic bonding is non-directional—that is, the magnitude of the bond is equal in all directions around an ion. Chapter 2 - 2
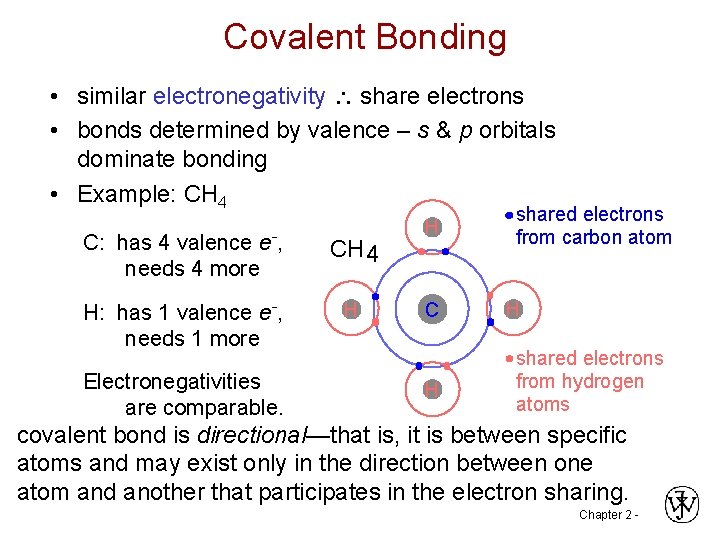
Covalent Bonding • similar electronegativity share electrons • bonds determined by valence – s & p orbitals dominate bonding • Example: CH 4 C: has 4 valence e-, needs 4 more CH 4 H: has 1 valence e-, needs 1 more H Electronegativities are comparable. H C H shared electrons from carbon atom H shared electrons from hydrogen atoms covalent bond is directional—that is, it is between specific atoms and may exist only in the direction between one atom and another that participates in the electron sharing. Chapter 2 -
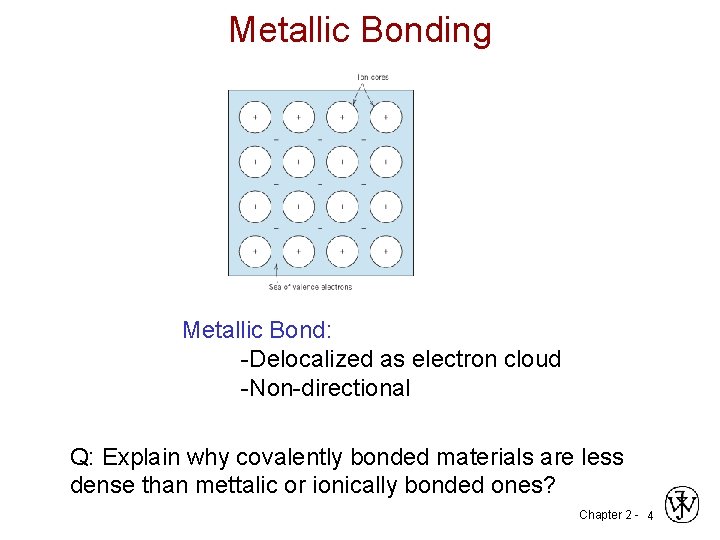
Metallic Bonding Metallic Bond: -Delocalized as electron cloud -Non-directional Q: Explain why covalently bonded materials are less dense than mettalic or ionically bonded ones? Chapter 2 - 4
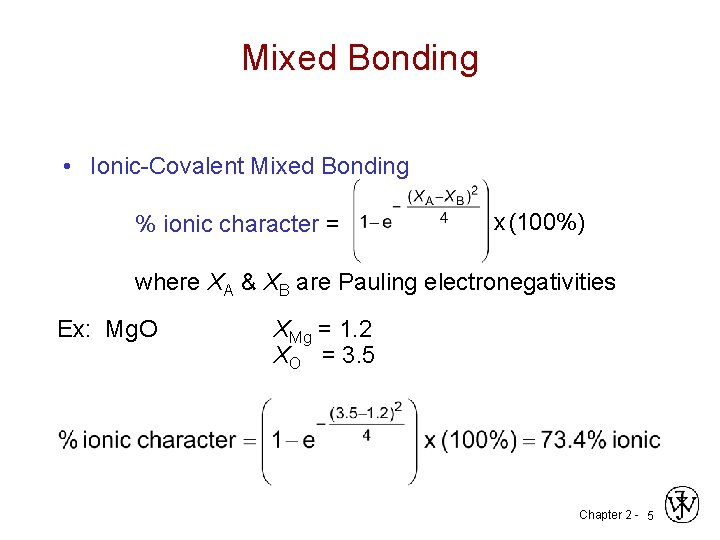
Mixed Bonding • Ionic-Covalent Mixed Bonding % ionic character = x (100%) where XA & XB are Pauling electronegativities Ex: Mg. O XMg = 1. 2 XO = 3. 5 Chapter 2 - 5
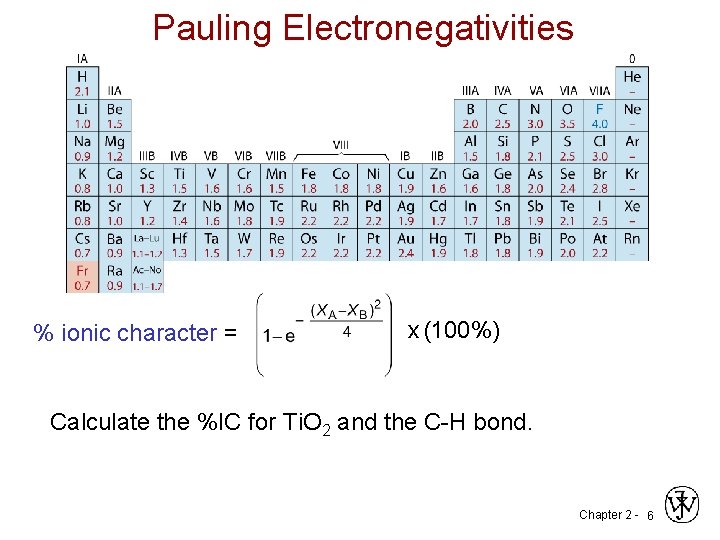
Pauling Electronegativities % ionic character = x (100%) Calculate the %IC for Ti. O 2 and the C-H bond. Chapter 2 - 6
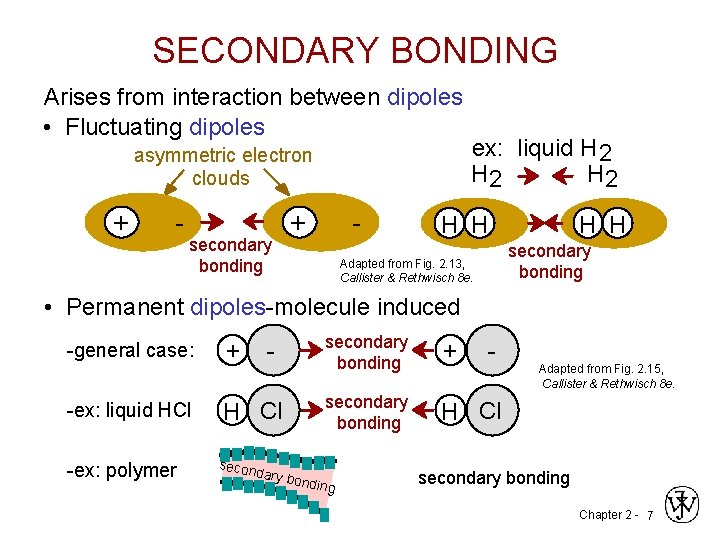
SECONDARY BONDING Arises from interaction between dipoles • Fluctuating dipoles asymmetric electron clouds + - secondary bonding + - ex: liquid H 2 H 2 H H secondary bonding Adapted from Fig. 2. 13, Callister & Rethwisch 8 e. • Permanent dipoles-molecule induced -general case: -ex: liquid HCl -ex: polymer + - H Cl secon dary b secondary bonding + secondary bonding H Cl ondin g - Adapted from Fig. 2. 15, Callister & Rethwisch 8 e. secondary bonding Chapter 2 - 7
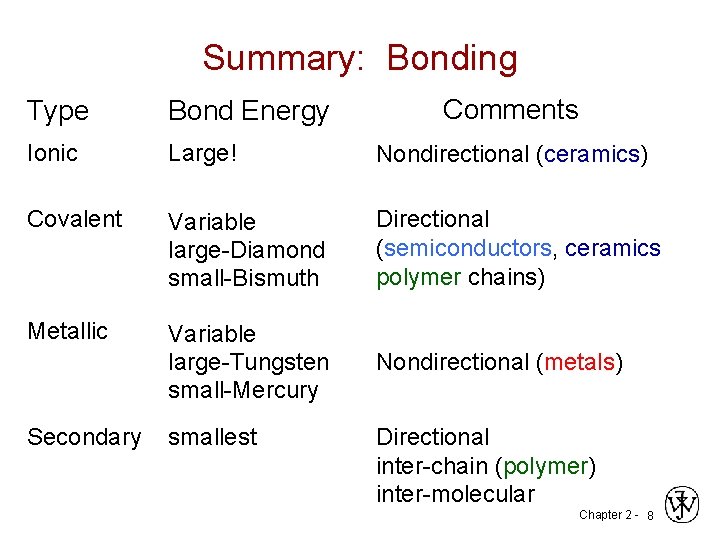
Summary: Bonding Comments Type Bond Energy Ionic Large! Nondirectional (ceramics) Covalent Variable large-Diamond small-Bismuth Directional (semiconductors, ceramics polymer chains) Metallic Variable large-Tungsten small-Mercury Nondirectional (metals) Secondary smallest Directional inter-chain (polymer) inter-molecular Chapter 2 - 8
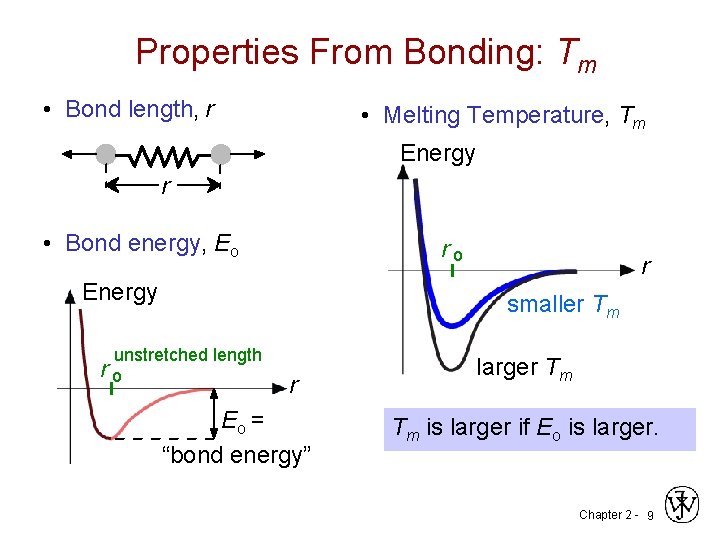
Properties From Bonding: Tm • Bond length, r • Melting Temperature, Tm Energy r • Bond energy, Eo ro Energy r smaller Tm unstretched length r o r Eo = “bond energy” larger Tm Tm is larger if Eo is larger. Chapter 2 - 9
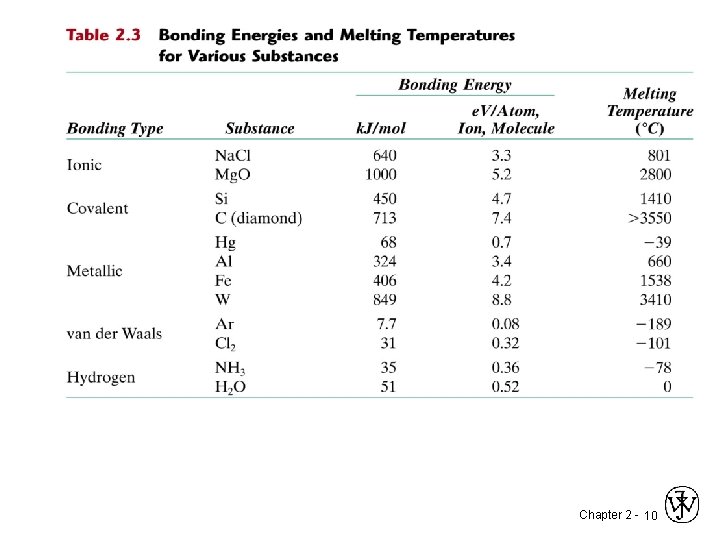
Chapter 2 - 10
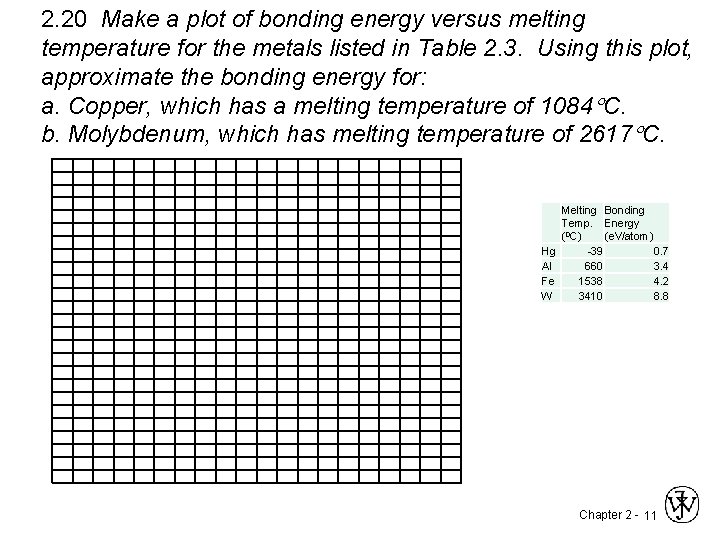
2. 20 Make a plot of bonding energy versus melting temperature for the metals listed in Table 2. 3. Using this plot, approximate the bonding energy for: a. Copper, which has a melting temperature of 1084 C. b. Molybdenum, which has melting temperature of 2617 C. Hg Al Fe W Melting Bonding Temp. Energy (0 C) (e. V/atom) -39 0. 7 660 3. 4 1538 4. 2 3410 8. 8 Chapter 2 - 11
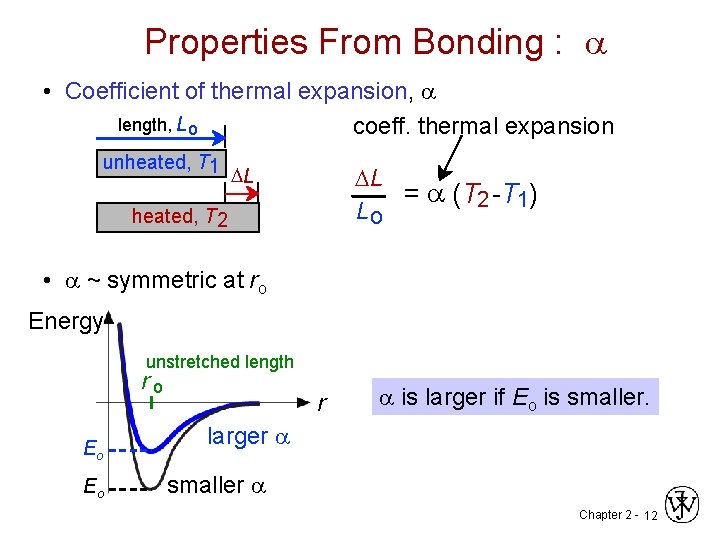
Properties From Bonding : a • Coefficient of thermal expansion, a length, L o coeff. thermal expansion unheated, T 1 DL DL = a (T 2 -T 1) Lo heated, T 2 • a ~ symmetric at ro Energy unstretched length ro Eo Eo r a is larger if Eo is smaller. larger a smaller a Chapter 2 - 12
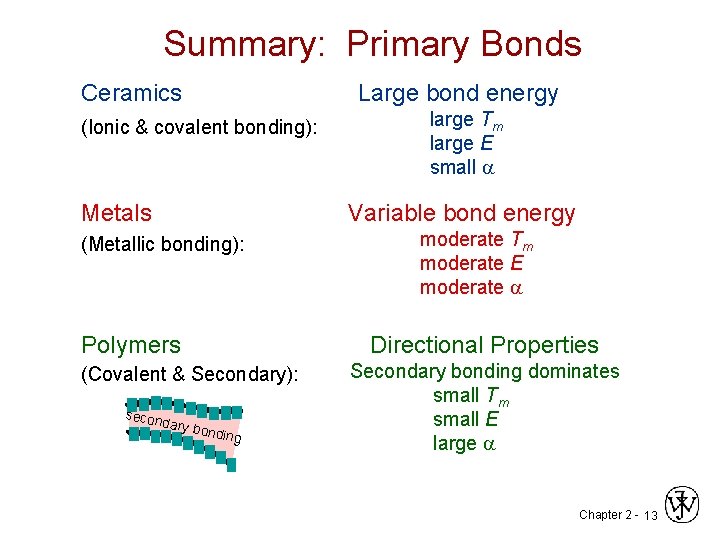
Summary: Primary Bonds Ceramics Large bond energy (Ionic & covalent bonding): Metals large Tm large E small a Variable bond energy (Metallic bonding): moderate Tm moderate E moderate a Polymers Directional Properties (Covalent & Secondary): Secondary bonding dominates small Tm small E large a secon dary b o nding Chapter 2 - 13
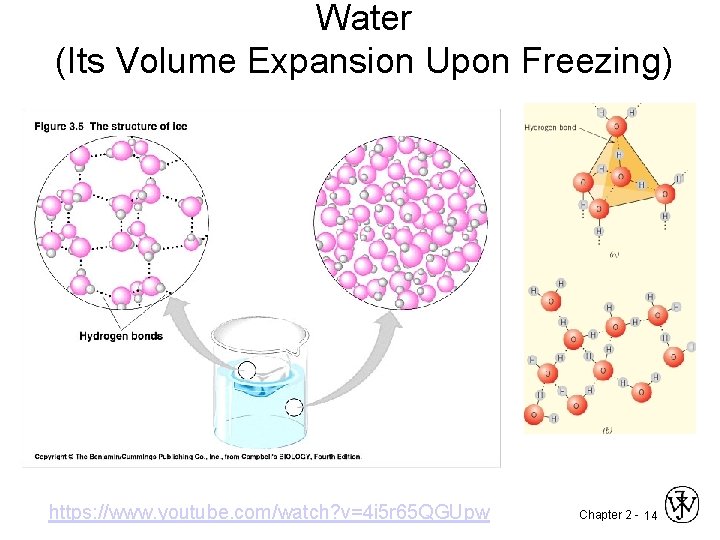
Water (Its Volume Expansion Upon Freezing) https: //www. youtube. com/watch? v=4 i 5 r 65 QGUpw Chapter 2 - 14
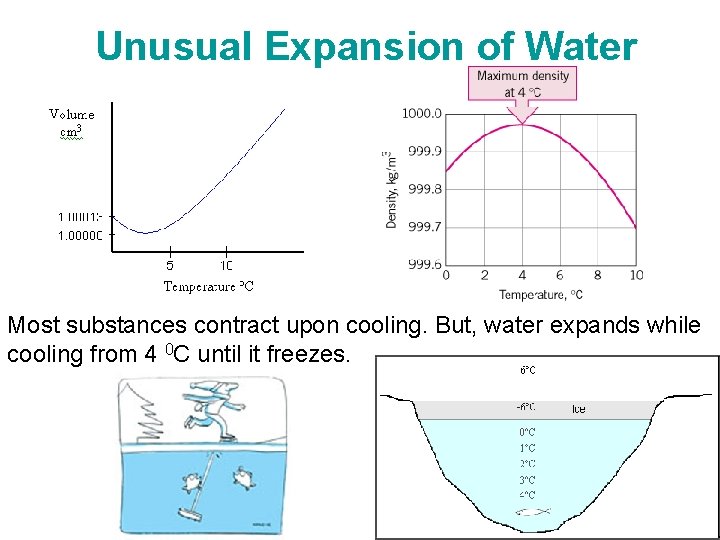
Unusual Expansion of Water Most substances contract upon cooling. But, water expands while cooling from 4 0 C until it freezes. Chapter 2 -
- Slides: 15