Ch 2 Classifying Matter Draw the following chart





















- Slides: 31

Ch 2 Classifying Matter

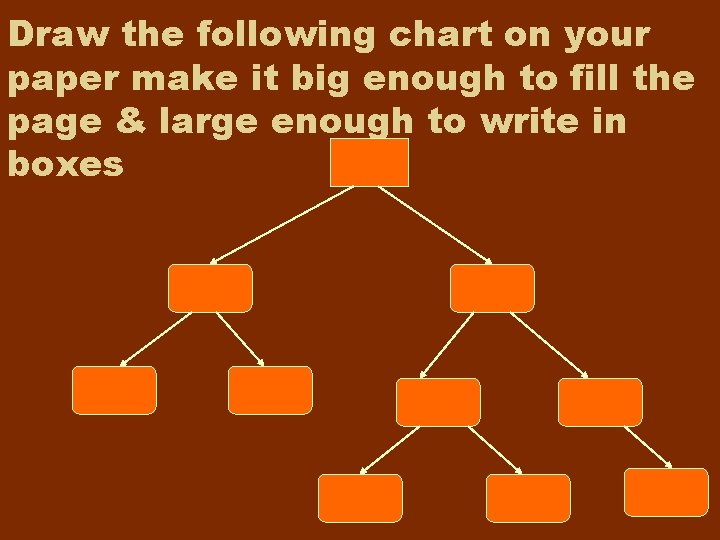
Draw the following chart on your paper make it big enough to fill the page & large enough to write in boxes

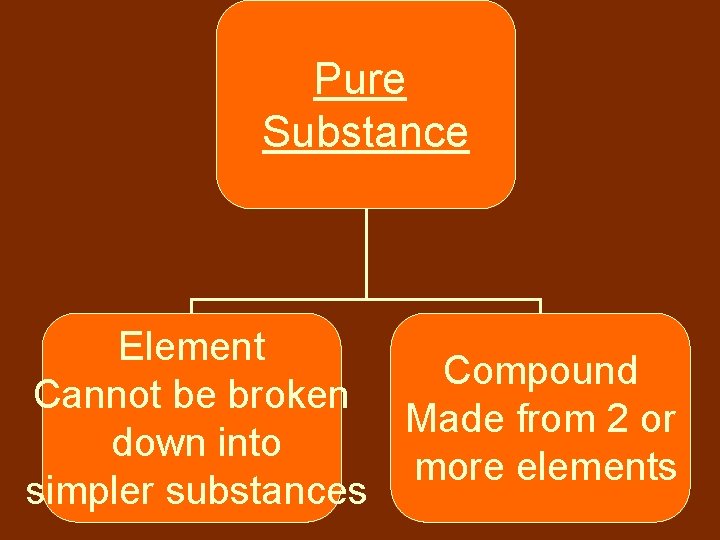
Matter Pure Substance Matter that always has same composition Mixtures Composition is not fixed

• Matter that always has exactly same composition ex: • salt • sugar • gold Pure Substance

Pure Substance Element Compound Cannot be broken Made from 2 or down into more elements simpler substances

Element • Atom—the smallest particle of an element • Ex: Carbon (C), Oxygen (O), Neon (Ne), Carbon (C), Platinum (Pt), Mercury (Hg)

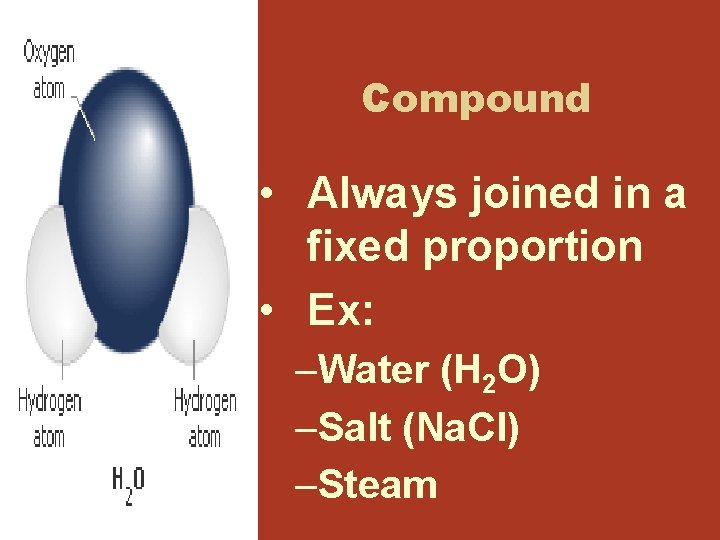
Compound • Always joined in a fixed proportion • Ex: –Water (H 2 O) –Salt (Na. Cl) –Steam

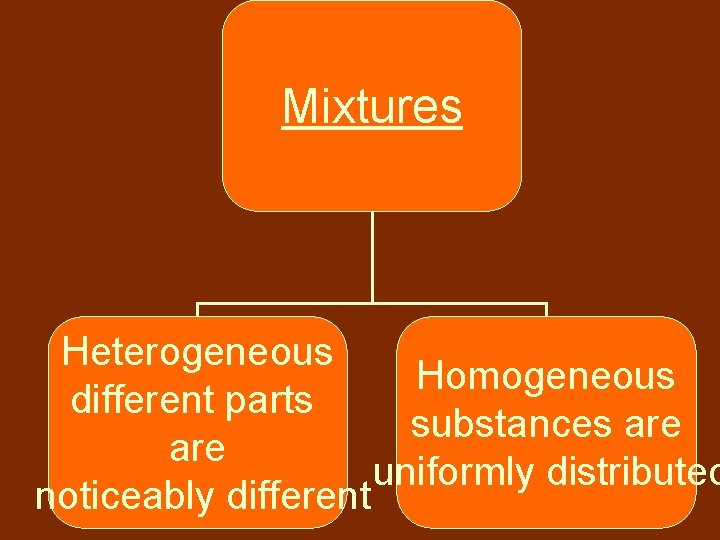
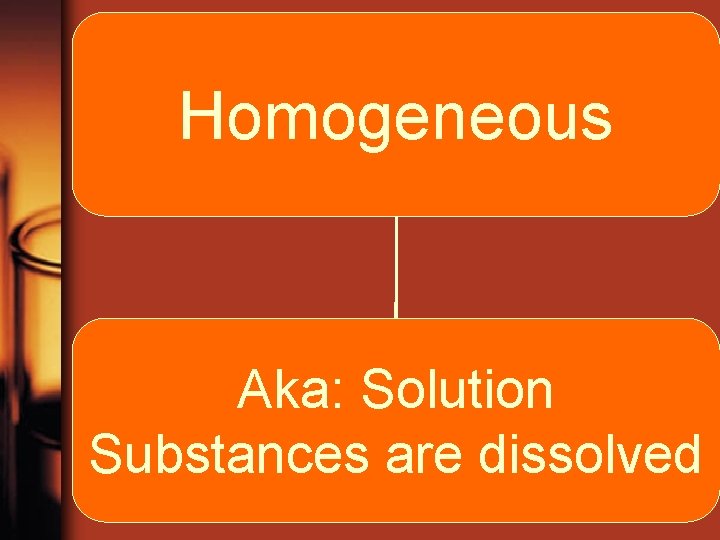
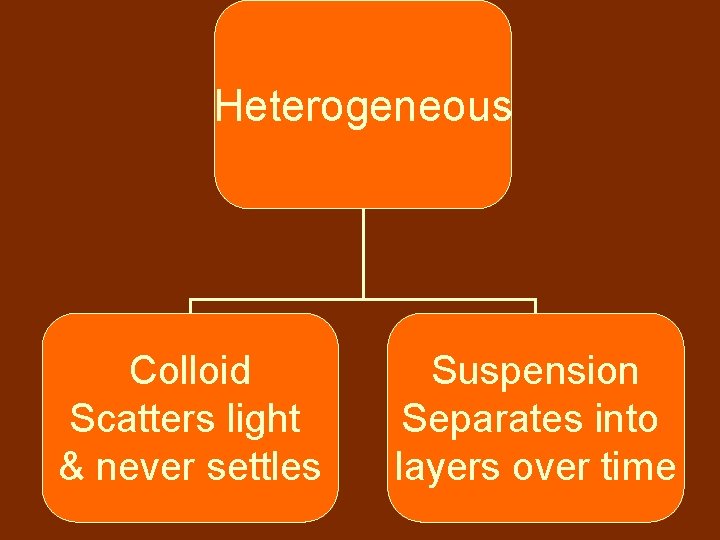
Mixtures Heterogeneous Homogeneous different parts substances are uniformly distributed noticeably different

Mixture-Homogeneous • Homogeneous mixtures contain substances that are uniformly spread out • aka: Solution • Ex: Vinegar, Syrup

Homogeneous Aka: Solution Substances are dissolved

Mixture-Heterogeneous • Heterogeneous mixtures have easily distinguished parts • Ex: – Pizza – Salsa – Chili

Heterogeneous Colloid Scatters light & never settles Suspension Separates into layers over time

Mixture-Heterogeneous Colloids scatter light & the particles never settle • Ex: Milk, smoke, fog Suspensions contain a liquid in which the visible particles settle • Ex: OJ, Italian Dressing • “shake it, shake it”

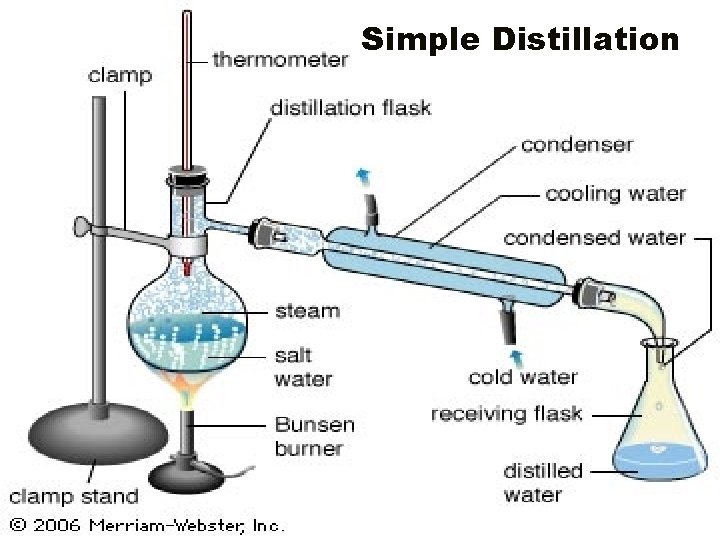
Separating mixtures • Filtration – process of separating a mixture based on particle size ex: coffee filter or air filter • Distillation – the process of separating a mixture based on the boiling points of the materials

Simple Distillation

Law of Conservation of Mass • Matter is neither created nor destroyed • mass of all substances present before a cc=mass of all substances remaining after change

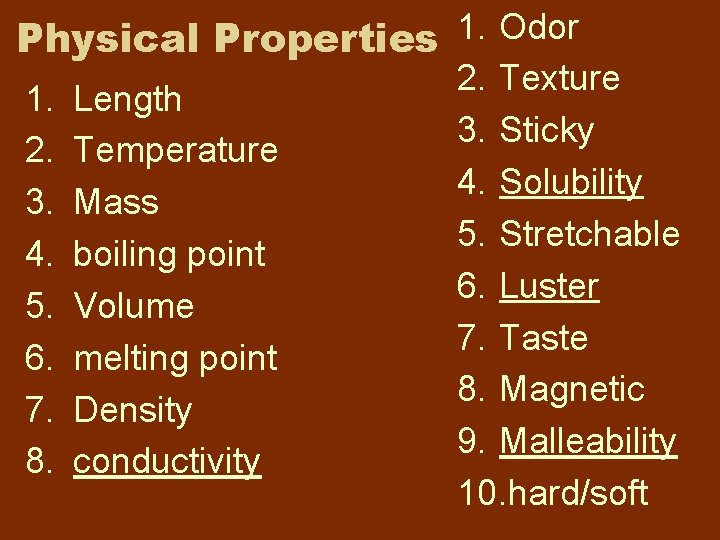
Physical Properties characteristic of a material you can observe w/o changing substances that make up the material

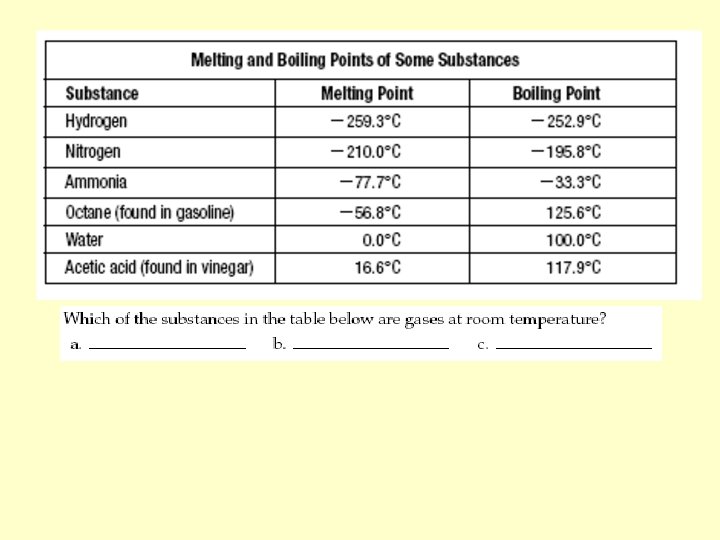
1. Odor Physical Properties 2. Texture 1. Length 3. Sticky 2. Temperature 4. Solubility 3. Mass 5. Stretchable 4. boiling point 6. Luster 5. Volume 7. Taste 6. melting point 8. Magnetic 7. Density 9. Malleability 8. conductivity 10. hard/soft

Physical Changes • change that does not alter the identity of substances in a material

Physical Changes • Ex 1. Breaking 2. Pounding 3. Cutting 4. Dissolving 5. Folding 6. Shredding 7. Crushing 8. Bending 9. Grinding 10. State Changes!? !

Chemical Properties-ability to undergo a chemical change • Ex: Flammability is the ABILITY to burn (property) not actually being on fire (change)

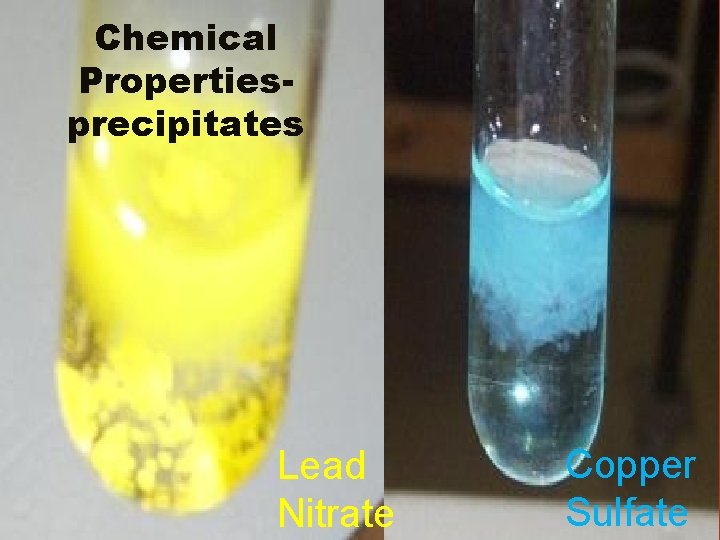
Chemical Properties • Reacts w/ light (fading) • Flammability • Reacts w/ oxygen – rust or tarnish • Reacts w/ water • Reacts w/ acid • Can form a precipitate

Chemical Propertiesprecipitates Lead Nitrate Copper Sulfate

PRECIPITATE VIDEO CLIPS • http: //sciencehack. com/videos/view/p. Fov l. Kp. PCb. I • http: //www. veoh. com/videos/v 273688 t. M XRPJ 7 q

Tarnished Copper Tarnished Silver Rust

Chemical Changes • change of a substance into new substances Ex 1. Rusting 2. burning 3. Tarnishing 4. formation of a precipitate

Chemical Changes *Signs or indicators –Fizzing –Bubbling –color change

Form-ing a precipitate

STAR Questions • List some indicators of a chemical change • Fizzing, bubbling, gas, heat, light, precipitate • Describe difference between property and change • Property is object’s ability to change, a change is action, it’s happening • What type of change is evaporating? • State changes = physical changes

____Photosynthesis ____Grinding meat into hamburger ____Has a sweet odor ____Drying clothes on a clothesline ____Flammable ____Digestion of food ____Growth of a plant ____Formation of clouds ____Dry ice subliming ____Length of 5 meters
