Ch 2 Atomic Structure I Subatomic Particles Development
Ch. 2 - Atomic Structure I. Subatomic Particles


Development of the Atom 1 st Model: Dalton’s Theory 1. All matter is made of indivisible atoms. 2. All atoms of a given element are identical. 2 nd Model: Thomson’s model “Plum-pudding” model. Discovered electron (negative charges) through Cathode Ray Tube Experiment. 3 rd Model: Rutherford’s Model. Discovered the nucleus made of protons through Gold Foil Experiment.

Development of the Atom ¨ 4 th Model: Bohr’s Model. “Planetary” model: Electrons orbit the nucleus on specific paths. ¨ Chadwick: Discovered the neutrons in the nucleus. Neutrons are the “glue” of the atom, or strong nuclear force.

Development of the Atom ¨ 5 th Model: Electron Cloud Model, the current model of the atom. Developed by Schrodinger (p. 96). Electrons are in “clouds” or regions of space outside the nucleus. Also called orbitals or energy levels.

Subatomic Particles ATOM Smallest particle that retains the chemical identity of an element ELECTRON CLOUD NUCLEUS PROTONS NEUTRONS ELECTRONS POSITIVE CHARGE NEUTRAL CHARGE NEGATIVE CHARGE Atoms Size Most of the atom’s mass.

Subatomic particles ¨ Atomic Number (Moseley): the # of protons in the nucleus of an atom • Unique to each element • For example: Oxygen: Atomic # 8 8 protons 8 electrons Positive charge = Negative charge in a neutral atom

Subatomic particles ¨ Mn (manganese) • # of protons: • # of electrons: ¨ Kr (Krypton) • # of protons: • # of electrons: 25 25 36 36

Mass Number ¨ mass # = protons + neutrons ¨ always a whole number – round the atomic mass ¨ NOT on the Periodic Table! © Addison-Wesley Publishing Company, Inc.

Mass Number ¨ Mass number – the atomic mass (weight) rounded to the nearest whole number ¨ Nuclear symbol: Mass # Atomic # ¨ Hyphen notation: carbon-12 Mass #

Ions ¨Ions – atoms with a net electrical charge (lose or gain one or more elctrons - # of protons is unchanged) • Oxygen O – 2 gained 2 e • Hydrogen H + lost 1 e • Magnesium Mg +2 or Mg 2+

Ions ¨ Write the symbol: • 9 protons, 10 electrons F-1 or F 1 - or F- • 13 protons, 10 electrons Al+3 or Al 3+ • S – 2 has how many protons and electrons? 16 p+ and 18 e-

Ions ¨ Iron has how many protons, electrons and neutrons? 56 Fe 26 26 protons, 26 electrons, 30 neutrons ¨ Fe+2 has how many protons, electrons, and neutrons? 26 protons, 24 electrons, 30 neutrons

Save for 2 nd Day ¨ Chlorine-37 - Write the nuclear symbol then find: • atomic #: 17 • mass #: 37 • # of protons: 17 • # of electrons: 17 • # of neutrons: 20

Day 2: Isotopes … Atoms of the same element with different mass numbers ( diff. # of neutrons ) …don’t have to be radioactive. Some isotopes are unstable and decay But, there are many stable isotopes that don’t decay.

Isotopes © Addison-Wesley Publishing Company, Inc.

Isotopes ¨ Hydrogen Isotopes: • Hydrogen-1 : 1 p, 1 e, 0 n (protium) • Hydrogen-2 : 1 p, 1 e, 1 n (deuterium) • Hydrogen-3 : 1 p, 1 e, 2 n (tritium)

Isotopes Some elements have several Isotopes Lead has four naturally occurring isotopes, Pb-204, Pb-206, Pb-207, and Pb-208; but there are 23 manmade isotopes of lead.

Isotopes ¨ The periodic table gives the average atomic mass of all the naturally occurring isotopes according to how abundant the isotope is. ¨ If the avg. atomic mass is rounded to the nearest whole number it will tell the most abundant isotope. • Carbon: 12. 0107 amu so…… • Hydrogen: 1. 00794 amu so….

Relative Atomic Mass ¨ 12 C atom = 1. 992 × 10 -23 g ¨ atomic mass unit (amu – Symbol: ) ¨ 1 amu = 1/12 the mass of a 12 C atom ¨ 1 amu = 1. 66 × 10 -24 g ¨ Carbon: 12. 0107 amu ¨ NOT 12. 0107 g !! © Addison-Wesley Publishing Company, Inc.

Average Atomic Mass ¨ weighted average of all isotopes ¨ on the Periodic Table (write this on p. t) ¨ round to 2 decimal places Avg. Atomic Mass

Average Atomic Mass ¨ EX: Calculate the avg. atomic mass of oxygen if its abundance in nature is: 99. 76% Oxygen-16; 0. 04% Oxygen-17; and 0. 20% Oxygen-18. Avg. Atomic Mass 16. 00 amu

Changes in the Nucleus ¨ Radioactivity: Unstable nuclei undergo spontaneous emission of radiation (particles or waves) from the nucleus of an atom ¨ Most atoms have stable nuclei – Not radioactive ! ¨ Atoms with unstable nuclei are radioactive !

Did you ever wonder. . . …why the nucleus stays together with all those positively charged protons in such a small space? Protons are all positive and … positive objects repel each other, – the nucleus shouldn’t exist!

Nuclear Decay • Protons do not repel each other because of a “strong nuclear force”, the neutrons, or the “glue”. • For some elements, there are not enough neutrons compared to all of the protons, to keep the nucleus from decaying. • In general elements 83 and larger are radioactive.

Three types of Radiation ¨Alpha particles - a ¨Beta particles -b ¨Gamma rays -g These were named by Ernest Rutherford.

Radiation ¨ Radiation can be detected by photographic film or a Geiger counter. ¨ We are protected against radiation by shielding and distance. ¨ Radiation is measured in units called mrems (millirems).

Radiation in the ground That radiation comes from the uranium beneath your feet. Uranium in the ground decays according to a decay series.
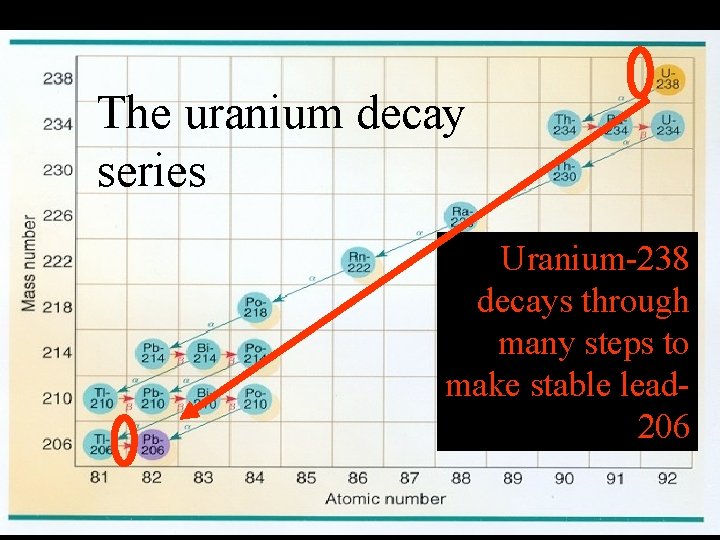
The uranium decay series Uranium-238 decays through many steps to make stable lead 206

The uranium decay series Locate radon – it will be important

Hazards from radon Radon is the only gas in the decay series of uranium. Radon gas works its way up through the ground and into your basements and crawl spaces.

Hazards from radon You breathe radon into your lungs. When a radon atom decays it releases an alpha particle … …which travels only a short distance before it is absorbed by your lungs, an transfers its energy.

Hazards from radon This ionizing radiation in your lungs can cause lung cancer. Smoking cigarettes and breathing radon really increases your chances of getting lung cancer.

Three types of radiation ¨ Alpha particle ( ) • helium nucleus Charge 2+ Stopped by: paper • 2 protons & 2 neutrons from nucleus ª Beta particle ( ) w Electron 1 - lead w Neutron in nucleus breaks w into proton and electron; electron is emitted ª Gamma ( ) w high-energy waves 0 concrete

Nuclear Decay ¨ Alpha Emission alpha particle Numbers must balance!!

Alpha decay

Alpha decay Radon-220 decays by alpha emission. What is the decay product? 86 Rn 220 4 2 He + 216 ? ? ? 84 Po

B. Nuclear Decay ¨ Beta Emission electron

Beta decay We know that neutrons decay into protons, which stay in the nucleus, and electrons, which are ejected from the nucleus as beta particles.

Beta decay Decay of a neutron: 1 H 1 + n 0 1 neutron proton 0 e -1 electron The electron ejected from the nucleus is a beta particle.

Beta decay Zn-62 decays by beta emission. What is the decay product? 30 Zn 62 -1 e + 0 62 ? ? ? 31 Ga

Nuclear Decay ¨ Why nuclides decay… • need stable ratio of neutrons to protons DECAY SERIES TRANSPARENCY

Half-life ¨ Half-life (t½) • Time required for half the atoms of a radioactive nuclide to decay. • Shorter half-life = less stable.


Half-life mf: final mass mi: initial mass n: # of half-lives

Half-life ª Fluorine-21 has a half-life of 5. 0 seconds. If you start with 25 g of fluorine-21, how many grams would remain after 60. 0 s? GIVEN: WORK: t½ = 5. 0 s mf = mi (½)n mi = 25 g mf = (25 g)(0. 5)12 mf = ? mf = 0. 0061 g total time = 60. 0 s n = 60. 0 s ÷ 5. 0 s =12
- Slides: 46