Ch 19 Chemical Bonds Stability in Bonding Types

Ch 19 Chemical Bonds Stability in Bonding Types of Bonds Writing Formulas and Naming Compounds

What are Compounds? • Compounds – 2 or more elements bonded together a. Physical Properties Change b. Chemical Properties Change Hydrogen - gas – highly explosive Oxygen – gas needed to live Water – liquid – sustains life,
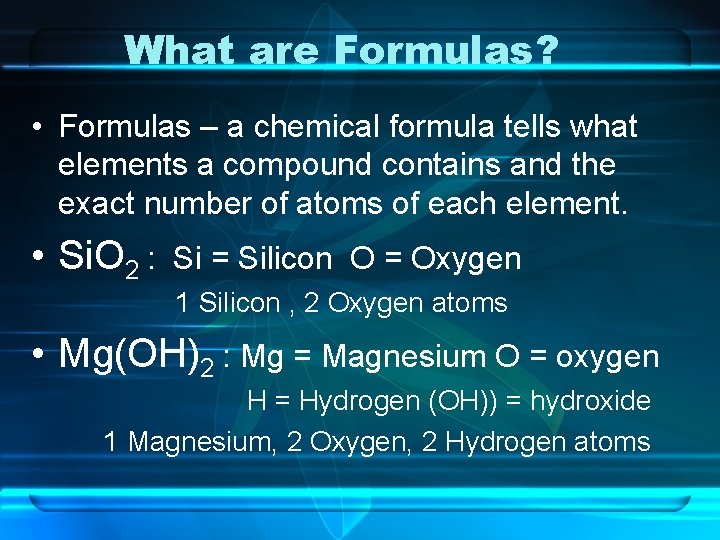
What are Formulas? • Formulas – a chemical formula tells what elements a compound contains and the exact number of atoms of each element. • Si. O 2 : Si = Silicon O = Oxygen 1 Silicon , 2 Oxygen atoms • Mg(OH)2 : Mg = Magnesium O = oxygen H = Hydrogen (OH)) = hydroxide 1 Magnesium, 2 Oxygen, 2 Hydrogen atoms

What is Atomic Stability? • Atoms are neutral, but not stable • Atoms form compounds to become stable. • Stable means that the outer energy level of electrons is full • The only group of atoms that are both neutral and stable are the Noble Gases (18)
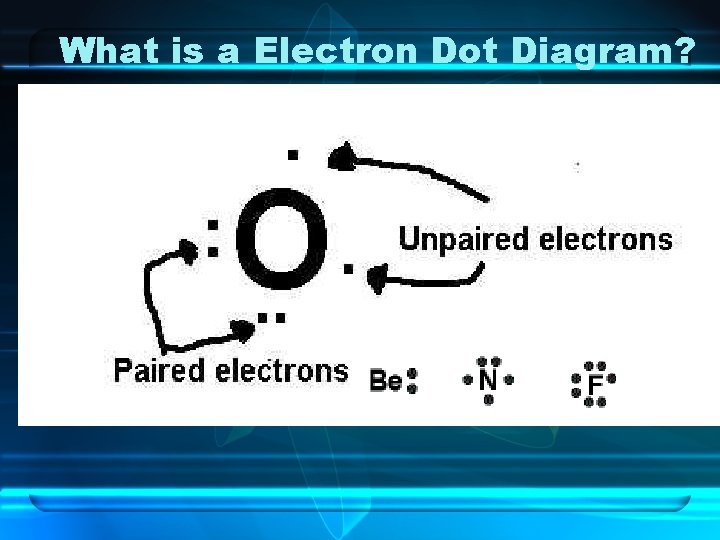
What is a Electron Dot Diagram?
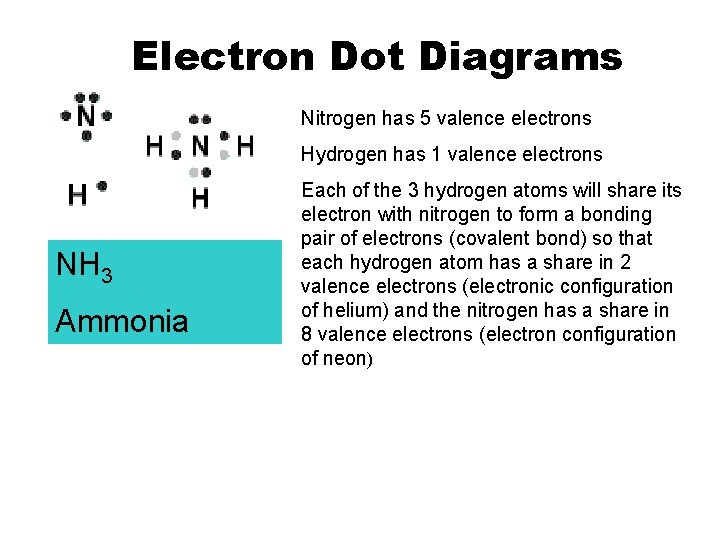
Electron Dot Diagrams Nitrogen has 5 valence electrons Hydrogen has 1 valence electrons NH 3 Ammonia Each of the 3 hydrogen atoms will share its electron with nitrogen to form a bonding pair of electrons (covalent bond) so that each hydrogen atom has a share in 2 valence electrons (electronic configuration of helium) and the nitrogen has a share in 8 valence electrons (electron configuration of neon)
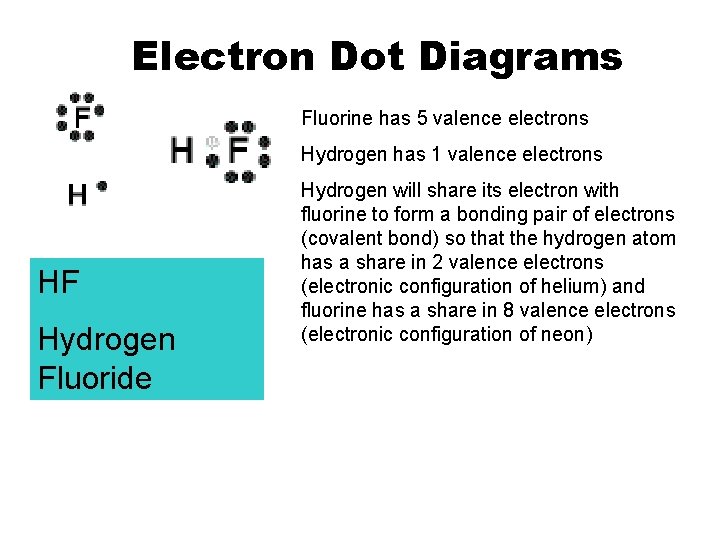
Electron Dot Diagrams Fluorine has 5 valence electrons Hydrogen has 1 valence electrons HF Hydrogen Fluoride Hydrogen will share its electron with fluorine to form a bonding pair of electrons (covalent bond) so that the hydrogen atom has a share in 2 valence electrons (electronic configuration of helium) and fluorine has a share in 8 valence electrons (electronic configuration of neon)
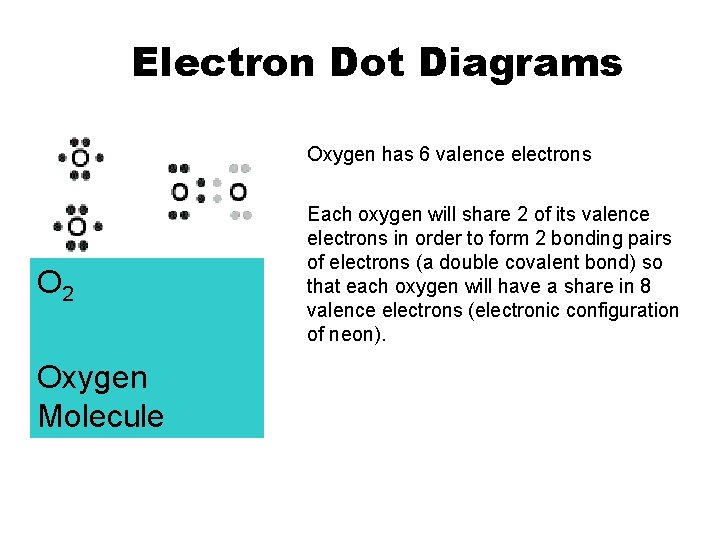
Electron Dot Diagrams Oxygen has 6 valence electrons O 2 Oxygen Molecule Each oxygen will share 2 of its valence electrons in order to form 2 bonding pairs of electrons (a double covalent bond) so that each oxygen will have a share in 8 valence electrons (electronic configuration of neon).
Types of Bonds • Two main types are a) Ionic and b) Covalent • Ionic bonds formed when atoms gain or lose electrons – this causes a force of attraction between oppositely charged atoms (ions). • When one element loses electrons, one or more other elements must gain an equal number of electrons • The net charge of an ionic compound is 0.
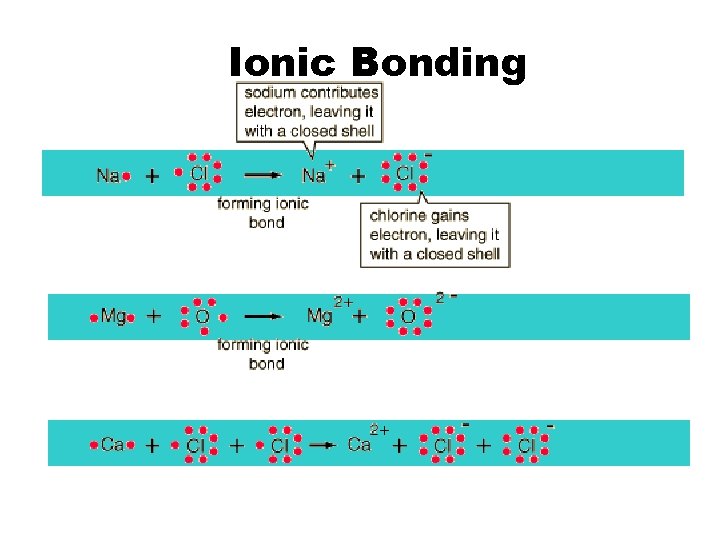
Ionic Bonding

Characteristics of Ionic Bonds Form crystalline solids High melting points and boiling points Conduct electricity when melted or dissolved in water Will not dissolve in non-polar liquid
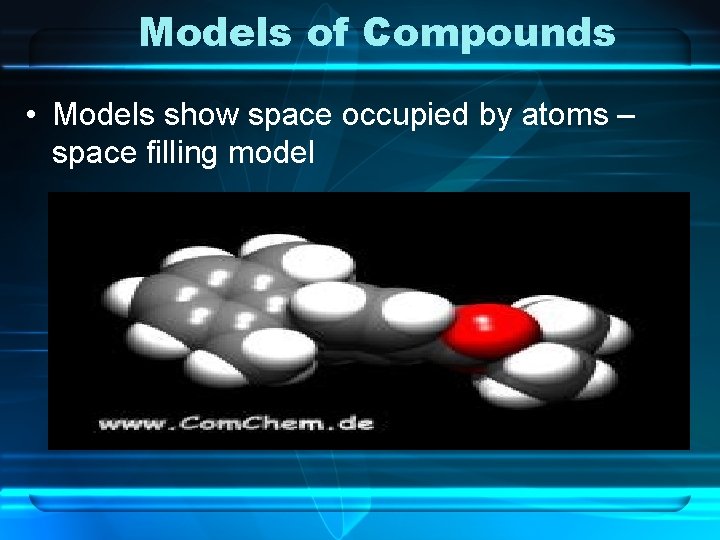
Models of Compounds • Models show space occupied by atoms – space filling model
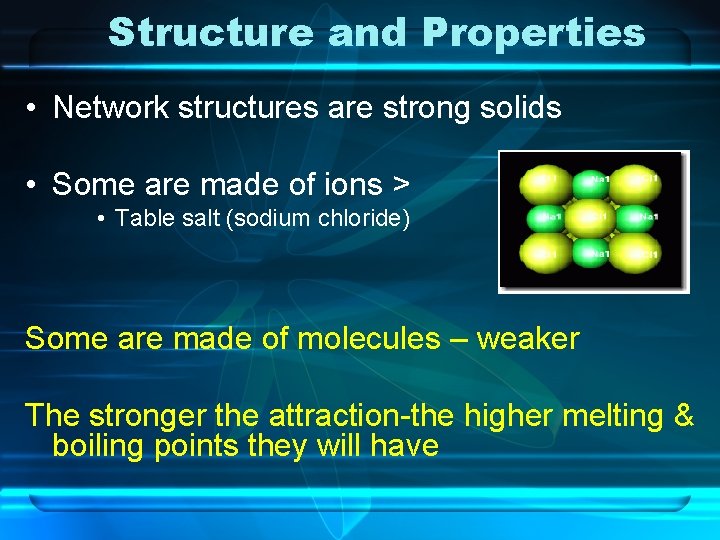
Structure and Properties • Network structures are strong solids • Some are made of ions > • Table salt (sodium chloride) Some are made of molecules – weaker The stronger the attraction-the higher melting & boiling points they will have

Ionic & Covalent Bonding • What holds bonded atoms together? • Ionic bonds – formed by transfer of electrons Ionic bonds are networks When dissolved in water – conduct electricity High melting and boiling points

Ionic & Covalent Bonding • Metallic bonds – all metals have these Metallic bonds are: flexible – can bend and stretch can conduct electricity • How? Packed close together, electrons overlap

Ionic & Covalent Bonding • Covalent bonds – atoms share electrons Covalent bonds are: not as strong as ionic bonds lower melting and boiling points • May share more than one pair of electrons • Electrons are not always shared equally Polar covalent bonds

Ionic & Covalent Bonding • Polyatomic Ions – covalently bonded atoms that act as a single ion

Names and Formulas • Naming Ionic compounds – – includes the ions of which they are made – some cations must show their charge – some have more than one oxidation state Transitions metals

Names and Formulas • Naming Covalent compounds – • Numerical prefixes are used 1 mono 6 hexa 2 di 7 hepta 3 tri 8 octa 4 tetra 9 nona 5 penta 10 deca-
Organic & Biochemical • Organic compounds – covalently bonded, made of molecules, contain carbon and almost always – hydrogen • Hydrocarbons – organic molecule made up of only hydrogen and carbon • Alkanes, Alkenes, Alcohols
Organic & Biochemical • Polymers – Large molecules, some natural and some are man made. Can have repeating subunits. • Examples – DNA, milk jugs(polyethene), ropes, carpet, artificial turf • Elasticity determined by structure – they are basically like long, thin chains (spaghetti).
Organic & Biochemical • Biochemical compounds – naturally occuring organic compounds that are very important to living things • Carbohydrates – sugars, starches, glycogen • Proteins – amino acids, insulin – sources are cheese, meats, eggs • DNA – very complex double helix structure. Every cell in your body has a copy
- Slides: 22