Ch 19 Acids and Bases Properties of Acids

Ch. 19 Acids and Bases

Properties of Acids 1. Sour taste 2. Change the color of acid-base indicators 3. React with metals to produce hydrogen gas 4. React with bases to produce salts and water 5. Some conduct electric current
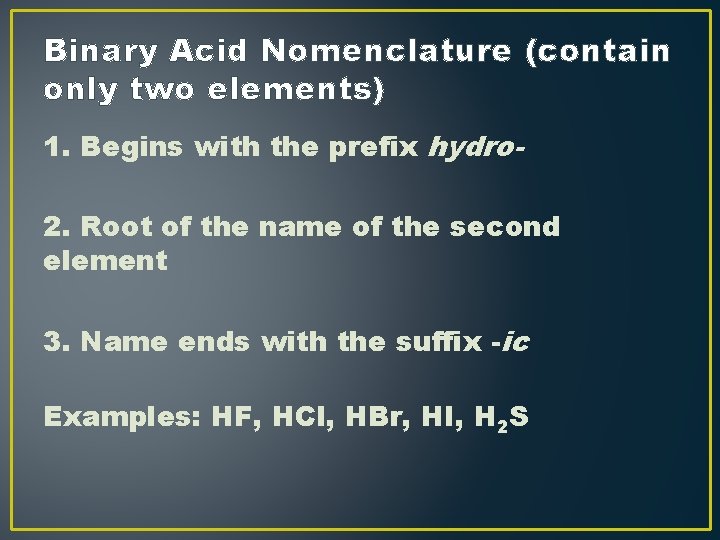
Binary Acid Nomenclature (contain only two elements) 1. Begins with the prefix hydro 2. Root of the name of the second element 3. Name ends with the suffix -ic Examples: HF, HCl, HBr, HI, H 2 S

Oxyacid • Definition: contains hydrogen, oxygen, and a third element • Examples: HIO 3, HCl. O 2, HNO 3
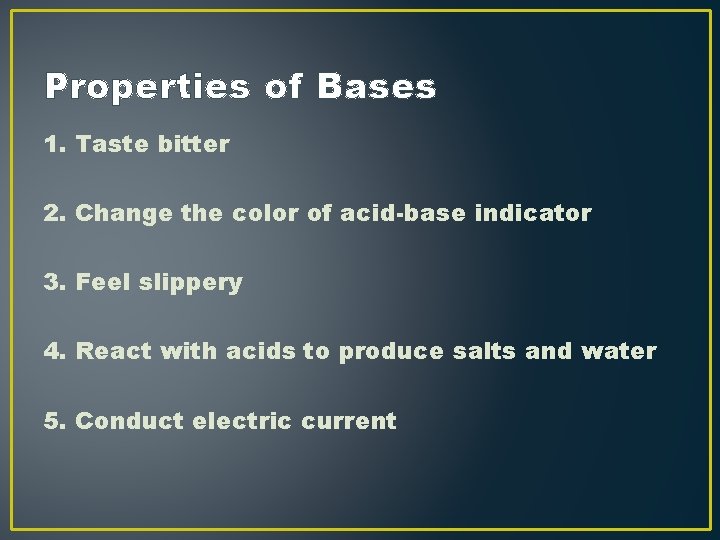
Properties of Bases 1. Taste bitter 2. Change the color of acid-base indicator 3. Feel slippery 4. React with acids to produce salts and water 5. Conduct electric current

Strong Acids • Definition: Ionize completely (H+ and anion) • 6 strong acids: (MEMORIZE) 1. 2. 3. 4. 5. 6. H 2 SO 4 HCl HNO 3 HBr HI

Weak Acids • Definition: do not ionize completely

Strong Bases • Definition: ionize completely (cation and OH-)

Weak Bases • Definition: do not ionize completely

Acid-Base Theories

Definitions • Arrhenius Acid • Arrhenius Base

Definitions • Bronsted-Lowry Acid • Bronsted-Lowry Base

Definitions • Lewis Acid • Lewis Base
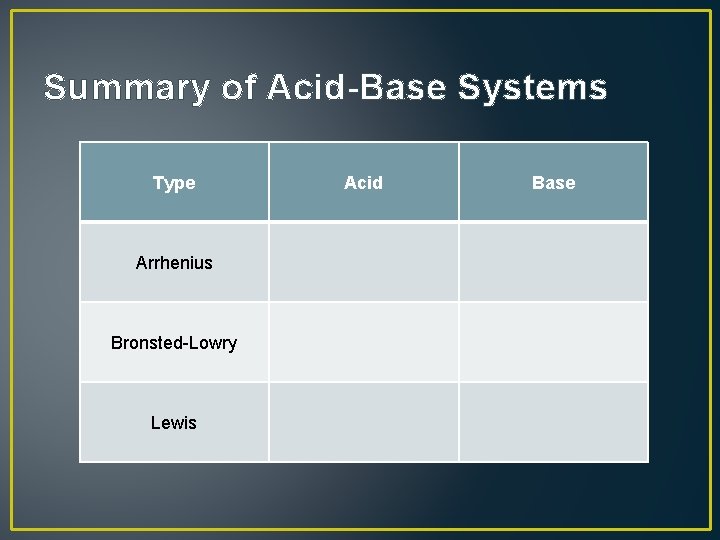
Summary of Acid-Base Systems Type Arrhenius Bronsted-Lowry Lewis Acid Base

Homework • P. 593 #3 -6 • Strong Acids Flashcards
- Slides: 15