Ch 17 Electrochemistry Electrochemistry Terminology 1 v Oxidation






- Slides: 23

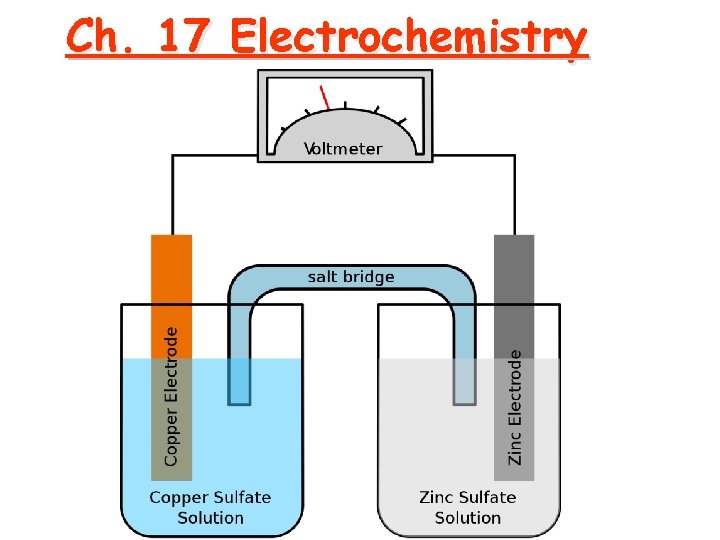
Ch. 17 Electrochemistry

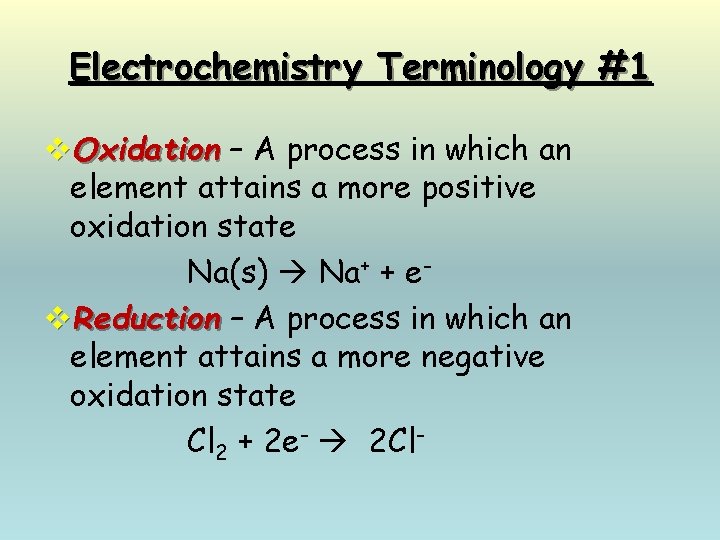
Electrochemistry Terminology #1 v. Oxidation – A process in which an element attains a more positive oxidation state Na(s) Na+ + ev. Reduction – A process in which an element attains a more negative oxidation state Cl 2 + 2 e- 2 Cl-

Electrochemistry Terminology #2 An old memory device for oxidation and reduction goes like this… LEO says GER Lose Electrons = Oxidation Gain Electrons = Reduction

Electrochemistry Terminology #3 q Oxidizing agent The substance that is reduced is the oxidizing agent q Reducing agent The substance that is oxidized is the reducing agent

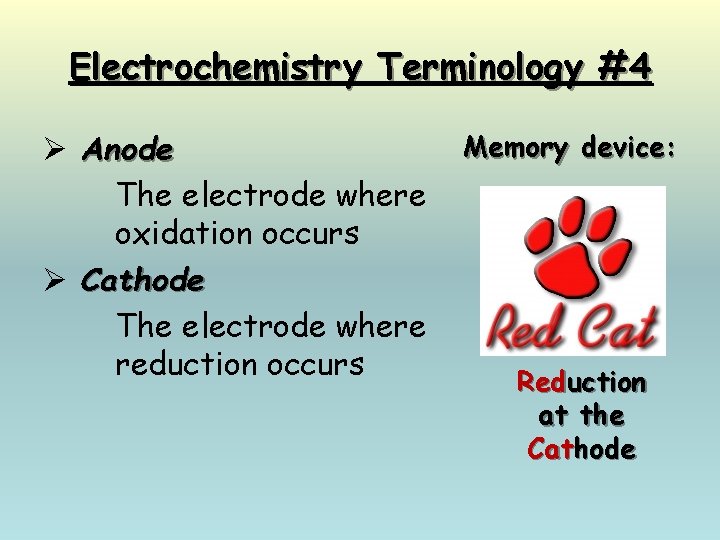
Electrochemistry Terminology #4 Ø Anode The electrode where oxidation occurs Ø Cathode The electrode where reduction occurs Memory device: Reduction at the Cathode

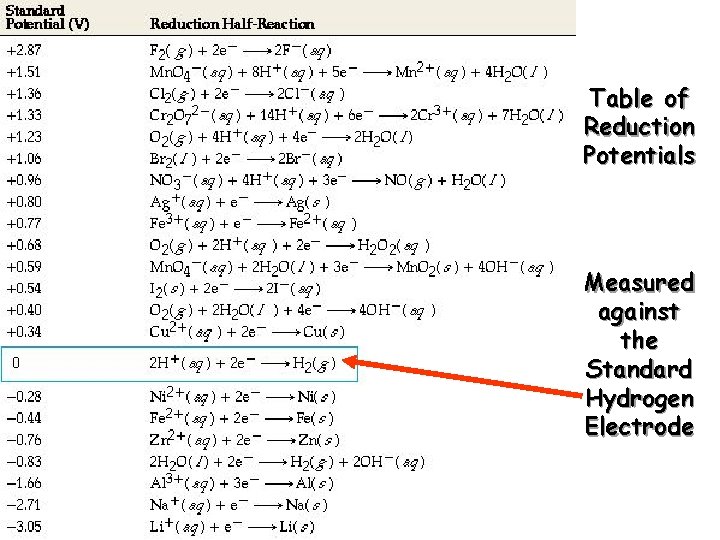
Table of Reduction Potentials Measured against the Standard Hydrogen Electrode

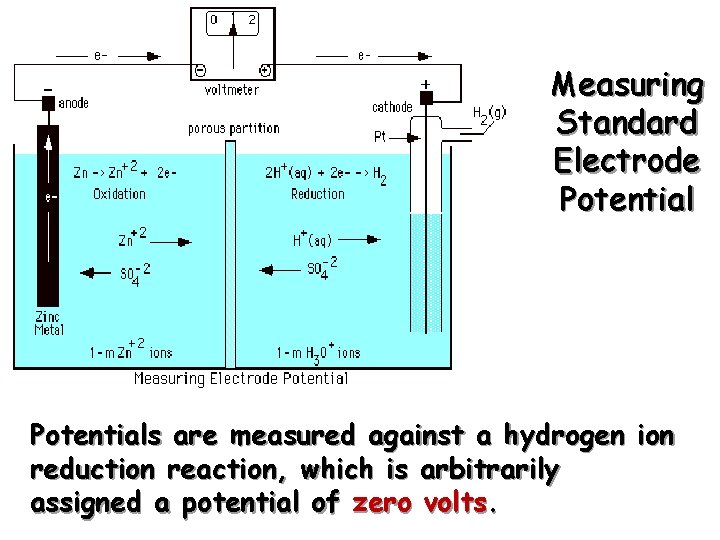
Measuring Standard Electrode Potentials are measured against a hydrogen ion reduction reaction, which is arbitrarily assigned a potential of zero volts.

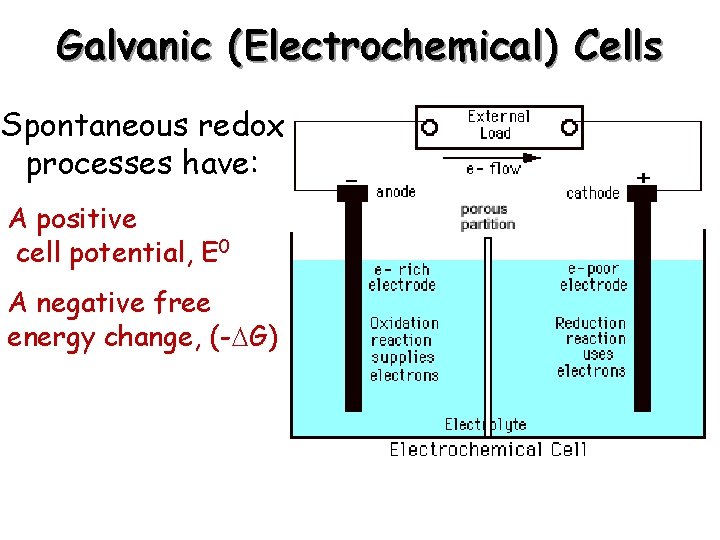
Galvanic (Electrochemical) Cells Spontaneous redox processes have: A positive cell potential, E 0 A negative free energy change, (- G)

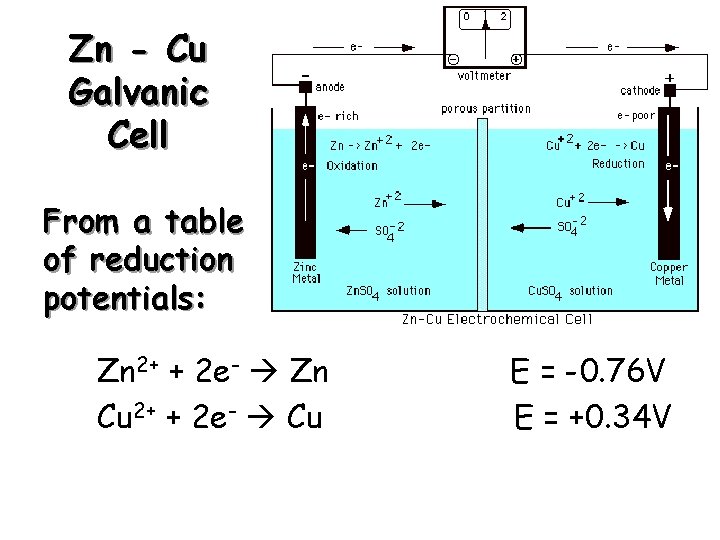
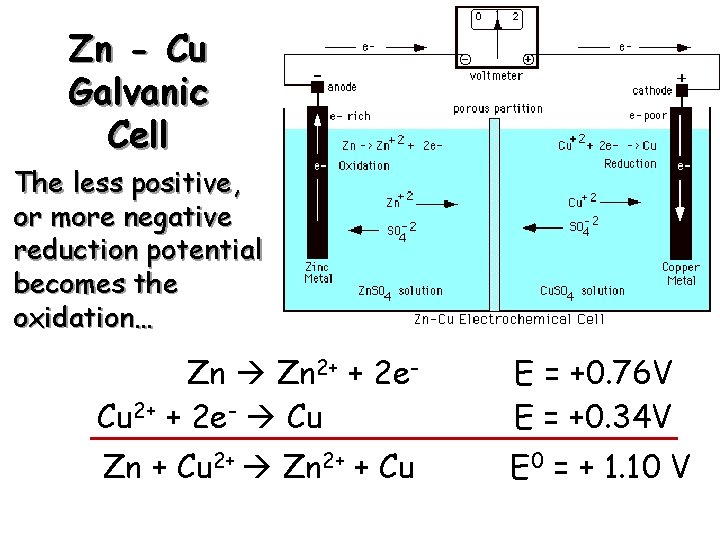
Zn - Cu Galvanic Cell From a table of reduction potentials: Zn 2+ + 2 e- Zn Cu 2+ + 2 e- Cu E = -0. 76 V E = +0. 34 V

Zn - Cu Galvanic Cell The less positive, or more negative reduction potential becomes the oxidation… Zn 2+ + 2 e. Cu 2+ + 2 e- Cu E = +0. 76 V E = +0. 34 V Zn + Cu 2+ Zn 2+ + Cu E 0 = + 1. 10 V

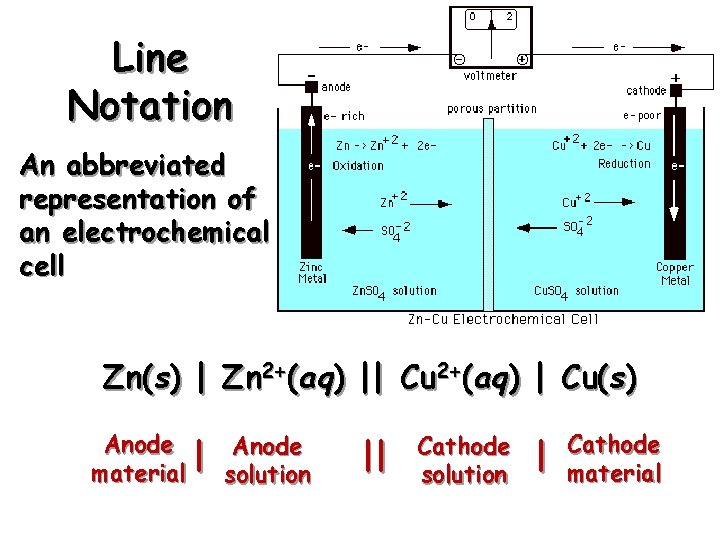
Line Notation An abbreviated representation of an electrochemical cell Zn(s) | Zn 2+(aq) || Cu 2+(aq) | Cu(s) Anode | material solution || Cathode solution | Cathode material

Agenda 1. 2. 3. 4. Catalyst Galvanic cells/Electrochemical cells Multiple Choice HW: read p. 836 -837

Consider the reduction potential chart p. 829 Find and copy the reduction equations for Ag+ Ag and Pb 2+ Pb. Be sure to include their reduction potentials (in volts). 1. Which metal ion has the greater reduction potential? 2. If these two metals (and their solutions) were used to create a galvanic cell, which metal would be the anode? 3. Write the reaction at the anode: 4. Write the reaction at the cathode:

5. What is the overall reaction? 6. What would be the voltage of the standard electrochemical cell? 7. If the concentration of Ag+ was increased, will the overall voltage increase or decrease? What if [Pb 2+] was increased? 8. Sketch the cell: 9. Write the cell notation for the cell: ______|________|_____

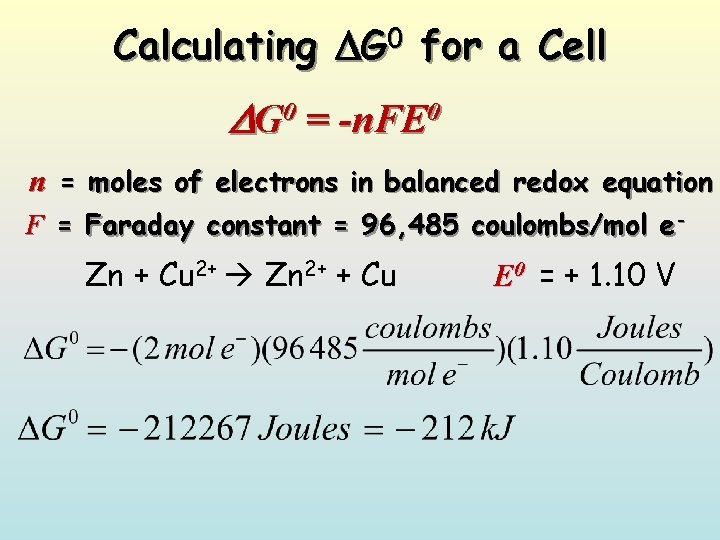
Calculating G 0 for a Cell G 0 = -n. FE 0 n = moles of electrons in balanced redox equation F = Faraday constant = 96, 485 coulombs/mol e- Zn + Cu 2+ Zn 2+ + Cu E 0 = + 1. 10 V

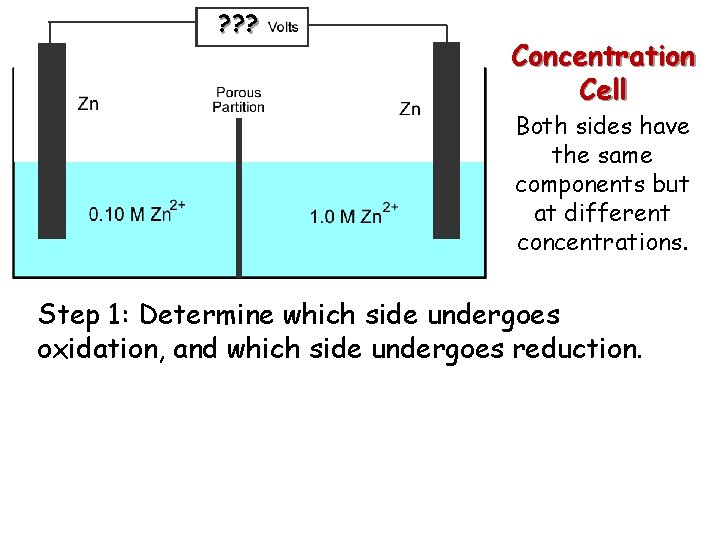
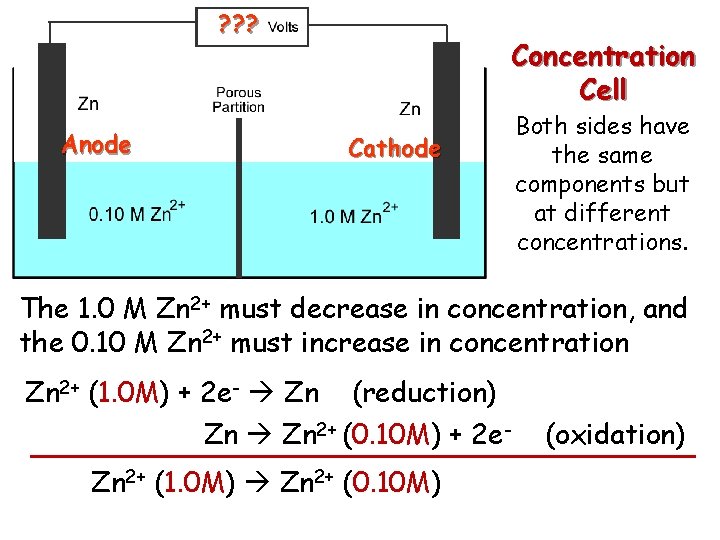
? ? ? Concentration Cell Both sides have the same components but at different concentrations. Step 1: Determine which side undergoes oxidation, and which side undergoes reduction.

? ? ? Anode Concentration Cell Cathode Both sides have the same components but at different concentrations. The 1. 0 M Zn 2+ must decrease in concentration, and the 0. 10 M Zn 2+ must increase in concentration Zn 2+ (1. 0 M) + 2 e- Zn (reduction) Zn 2+ (0. 10 M) + 2 e. Zn 2+ (1. 0 M) Zn 2+ (0. 10 M) (oxidation)

CW: Multiple Choice and FRQ a • Given 3 or more half-cells, how can you find the cell potential of a cell? • HW: Read p. 847 -851

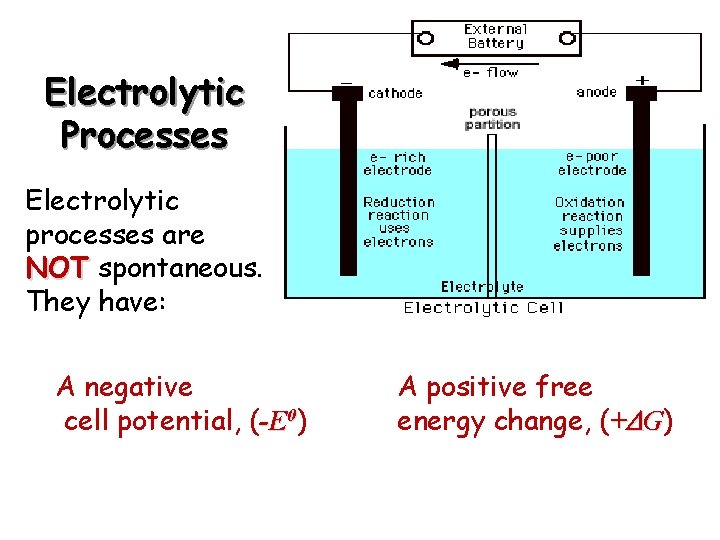
Electrolytic Processes Electrolytic processes are NOT spontaneous. They have: A negative cell potential, (-E 0) A positive free energy change, (+ G)

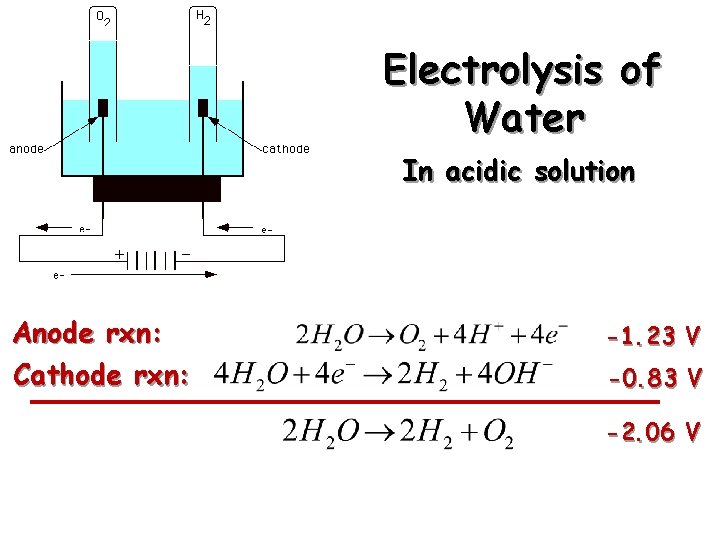
Electrolysis of Water In acidic solution Anode rxn: Cathode rxn: -1. 23 V -0. 83 V -2. 06 V

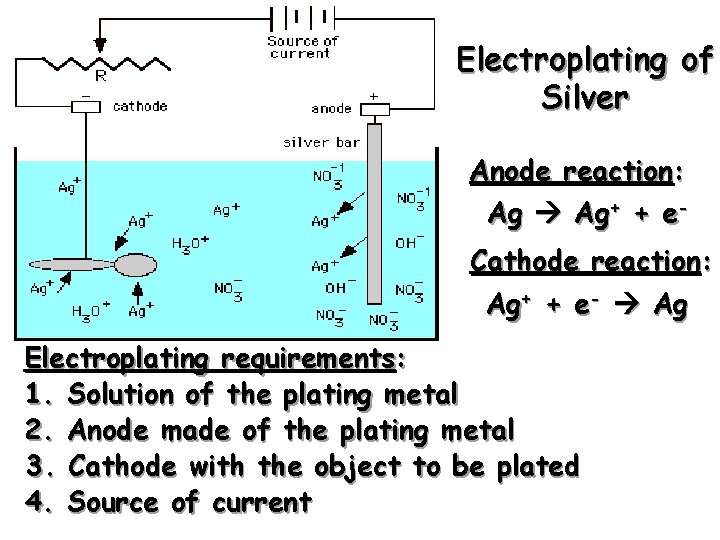
Electroplating of Silver Anode reaction: Ag Ag+ + e. Cathode reaction: Ag+ + e- Ag Electroplating requirements: 1. Solution of the plating metal 2. Anode made of the plating metal 3. Cathode with the object to be plated 4. Source of current

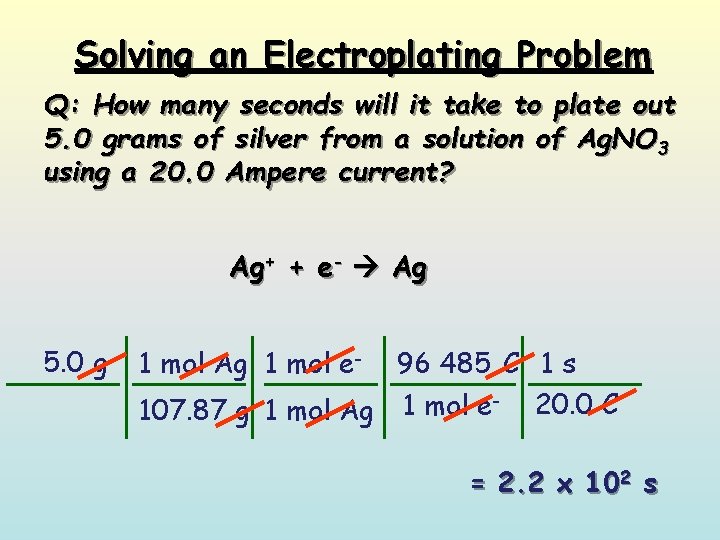
Solving an Electroplating Problem Q: How many seconds will it take to plate out 5. 0 grams of silver from a solution of Ag. NO 3 using a 20. 0 Ampere current? Ag+ + e- Ag 5. 0 g 1 mol Ag 1 mol e- 96 485 C 1 s 20. 0 C 1 mol e 107. 87 g 1 mol Ag = 2. 2 x 102 s

CW: FRQ and p. 866 #89 b-c & 91