CH 16 Chemistry of Benzene Renee Y Becker

CH 16: Chemistry of Benzene Renee Y. Becker CHM 2211 Valencia Community College 1
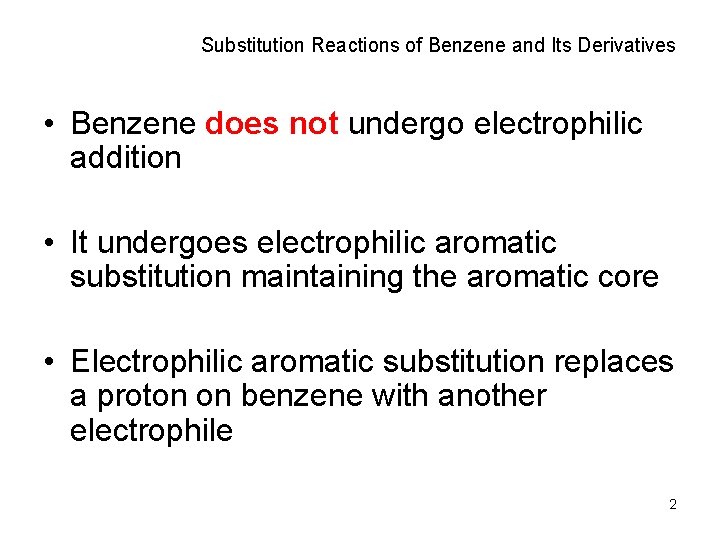
Substitution Reactions of Benzene and Its Derivatives • Benzene does not undergo electrophilic addition • It undergoes electrophilic aromatic substitution maintaining the aromatic core • Electrophilic aromatic substitution replaces a proton on benzene with another electrophile 2
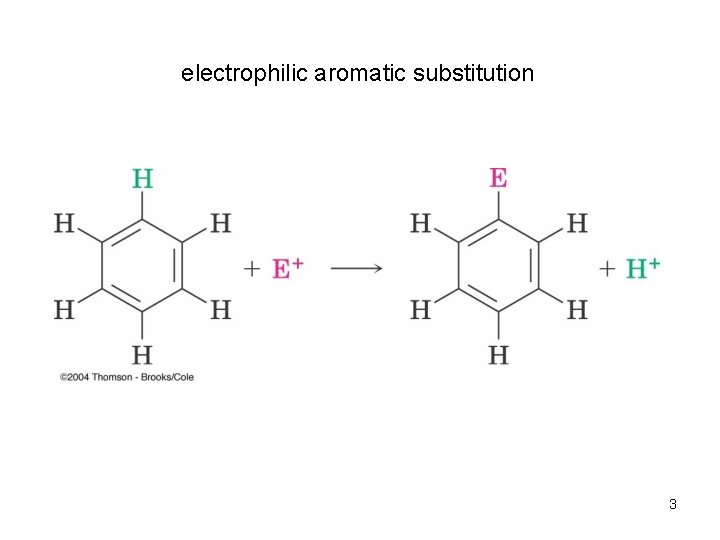
electrophilic aromatic substitution 3
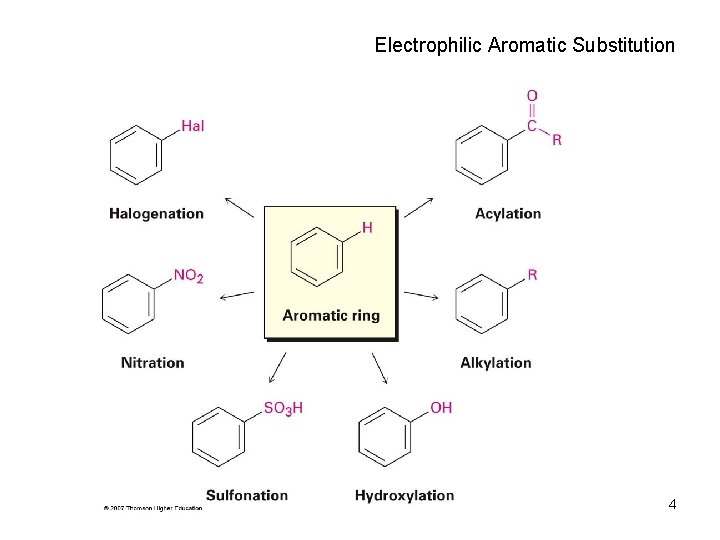
Electrophilic Aromatic Substitution 4

Halogenation of Benzene • Benzene’s electrons participate as a Lewis base in reactions with Lewis acids – Lewis acid: electron pair acceptor – Lewis base: electron pair donor • The product is formed by loss of a proton, which is replaced by a halogen 5

Bromination of Aromatic Rings • Benzene’s electrons participate as a Lewis base in reactions with Lewis acids • The product is formed by loss of a proton, which is replaced by bromine • Fe. Br 3 is added as a catalyst to polarize the bromine reagent 6
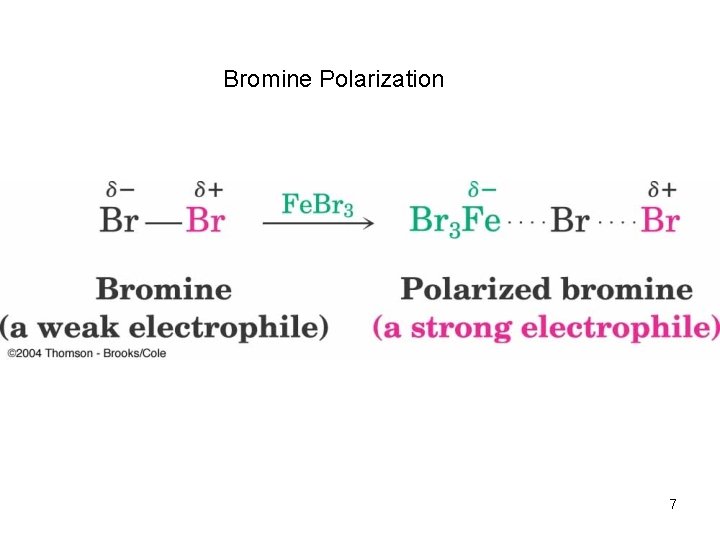
Bromine Polarization 7

Mechanism 1 • Diagram the mechanism for the bromination of benzene and note the formation of the carbocation: 8

Example 1 • Draw and name three possible products of the bromination of toluene (not including HBr). 9
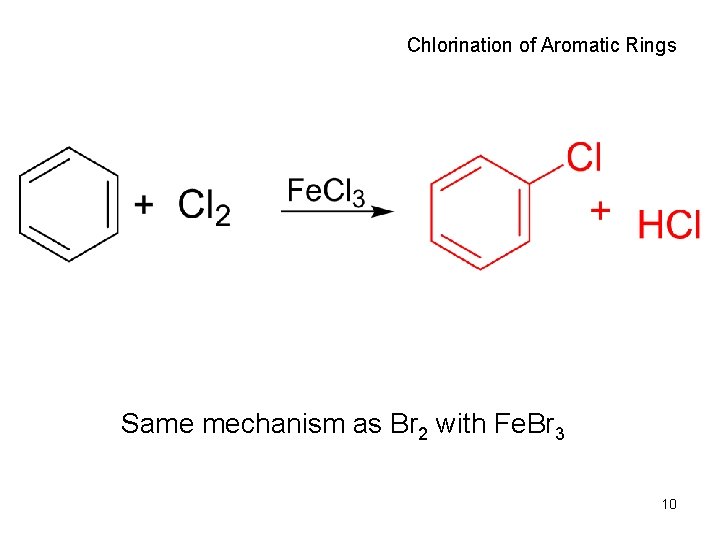
Chlorination of Aromatic Rings Same mechanism as Br 2 with Fe. Br 3 10
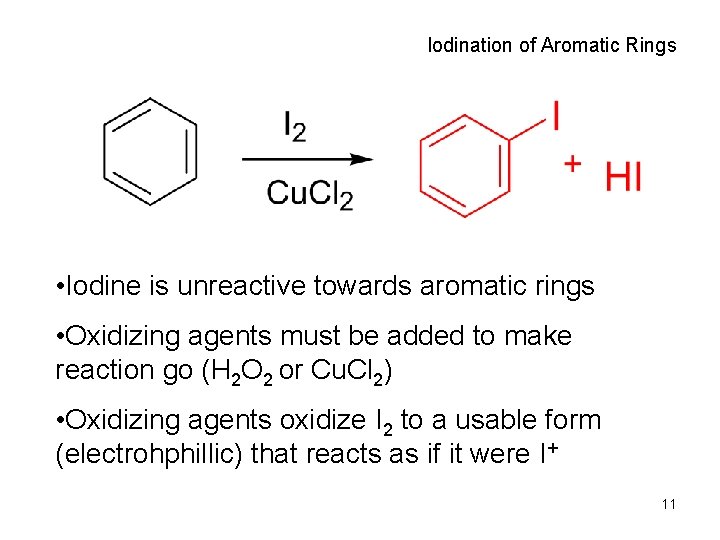
Iodination of Aromatic Rings • Iodine is unreactive towards aromatic rings • Oxidizing agents must be added to make reaction go (H 2 O 2 or Cu. Cl 2) • Oxidizing agents oxidize I 2 to a usable form (electrohphillic) that reacts as if it were I+ 11
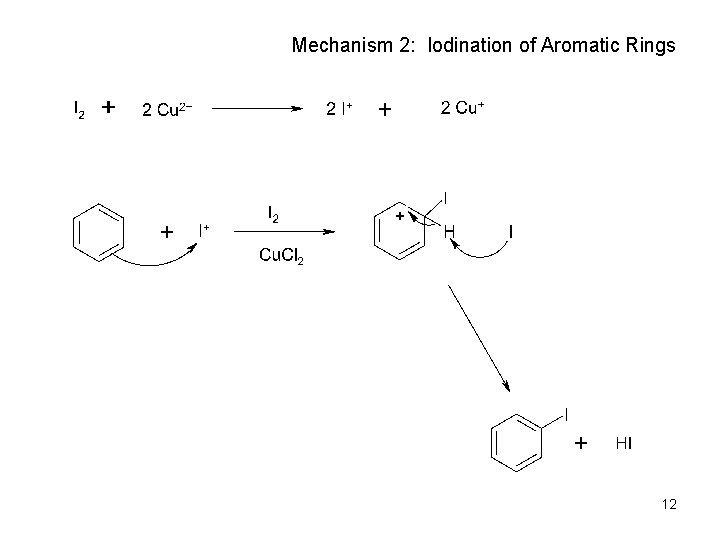
Mechanism 2: Iodination of Aromatic Rings 12
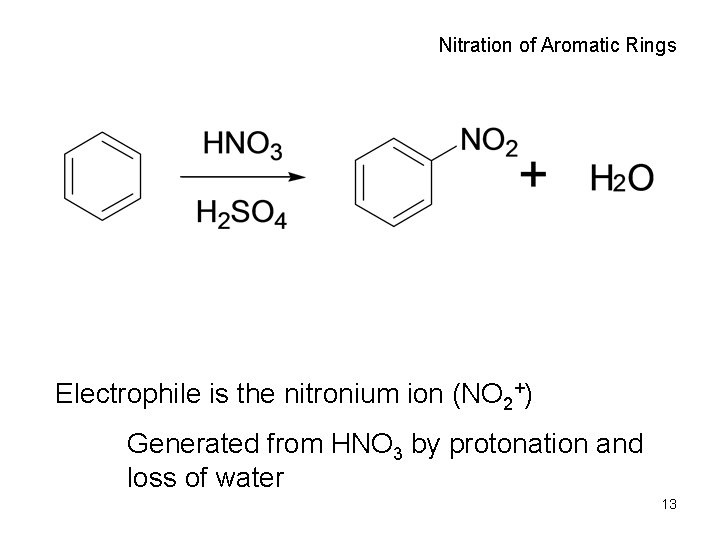
Nitration of Aromatic Rings Electrophile is the nitronium ion (NO 2+) Generated from HNO 3 by protonation and loss of water 13
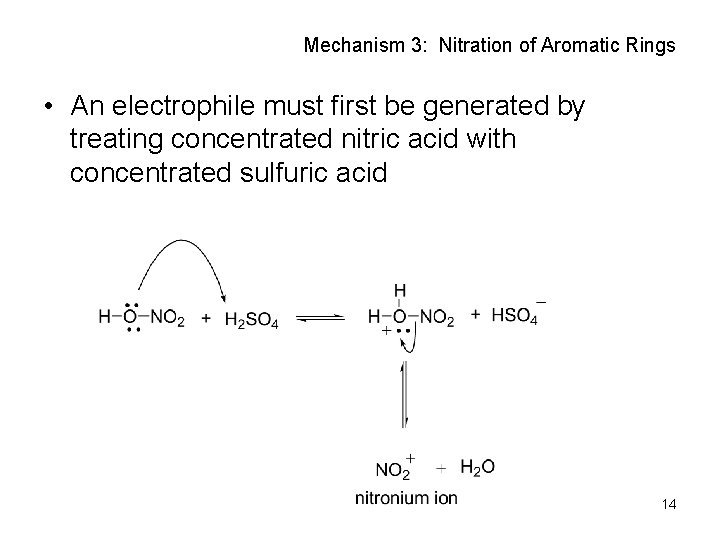
Mechanism 3: Nitration of Aromatic Rings • An electrophile must first be generated by treating concentrated nitric acid with concentrated sulfuric acid 14
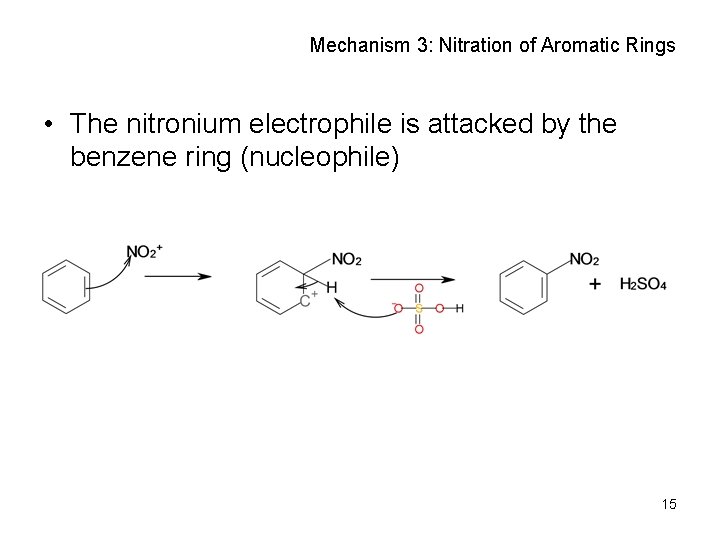
Mechanism 3: Nitration of Aromatic Rings • The nitronium electrophile is attacked by the benzene ring (nucleophile) 15
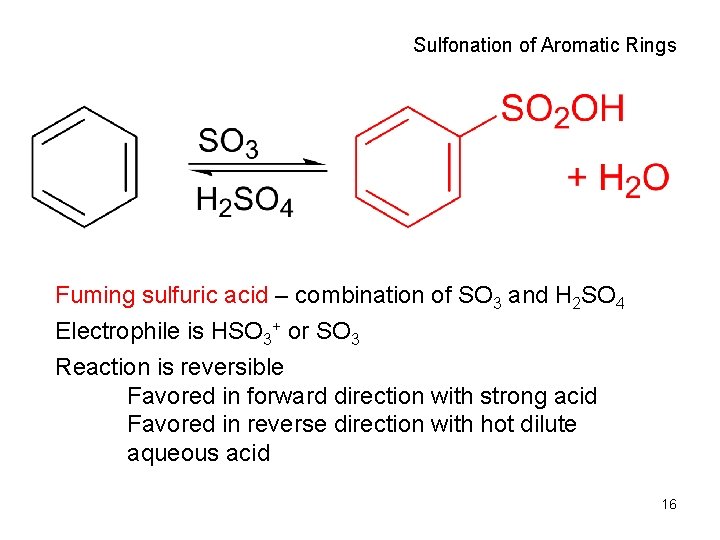
Sulfonation of Aromatic Rings Fuming sulfuric acid – combination of SO 3 and H 2 SO 4 Electrophile is HSO 3+ or SO 3 Reaction is reversible Favored in forward direction with strong acid Favored in reverse direction with hot dilute aqueous acid 16
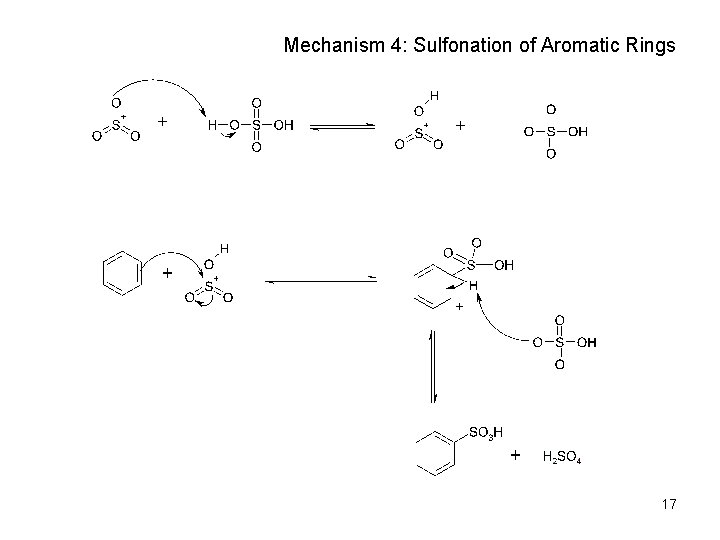
Mechanism 4: Sulfonation of Aromatic Rings 17
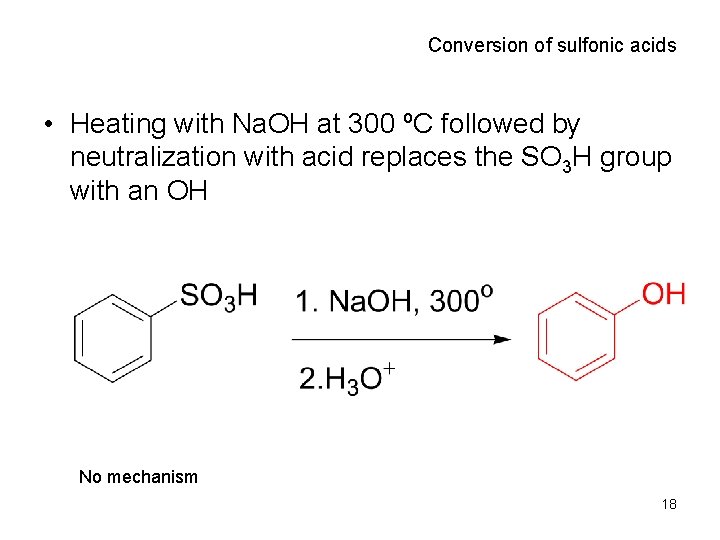
Conversion of sulfonic acids • Heating with Na. OH at 300 ºC followed by neutralization with acid replaces the SO 3 H group with an OH No mechanism 18
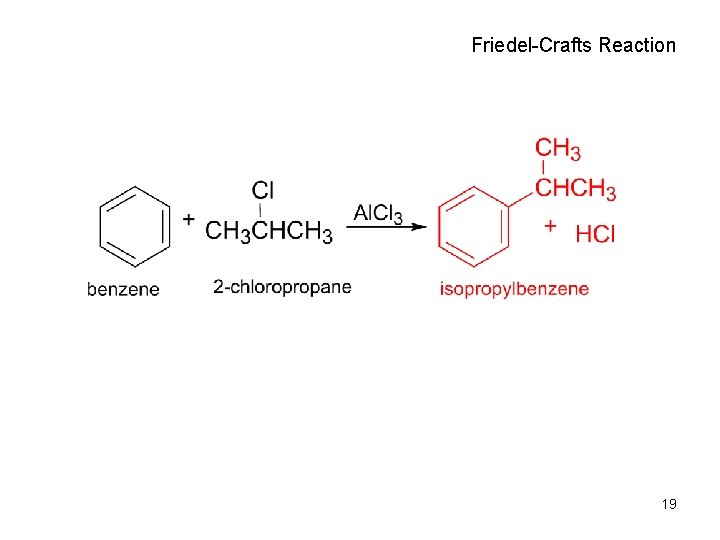
Friedel-Crafts Reaction 19
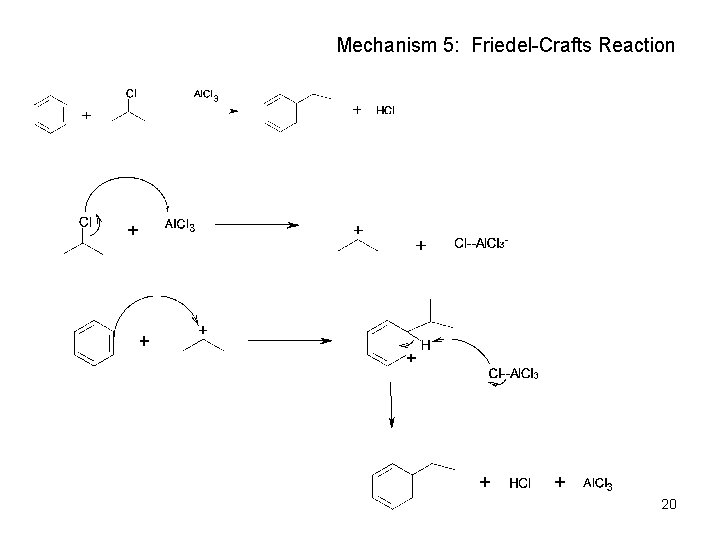
Mechanism 5: Friedel-Crafts Reaction 20

Friedel-Crafts Reaction (Alkylation of Aromatic Rings) • the electrophile is a carbocation, R+ • only alkyl halides can be used – aryl halides and vinylic halides do not react. • will not occur on aromatic rings substituted by electron withdrawing substituents • can’t eat just one! It’s hard to stop after one substitution • skeletal rearrangements of the alkyl group often occur when using primary alkyl halides 21
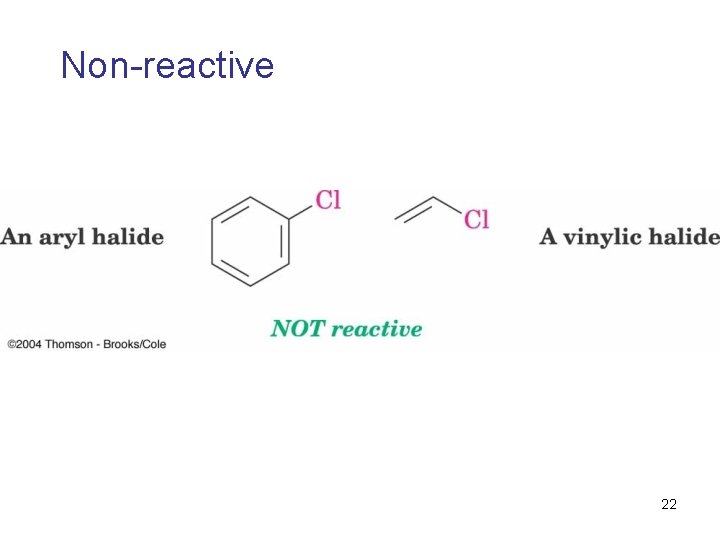
Non-reactive 22
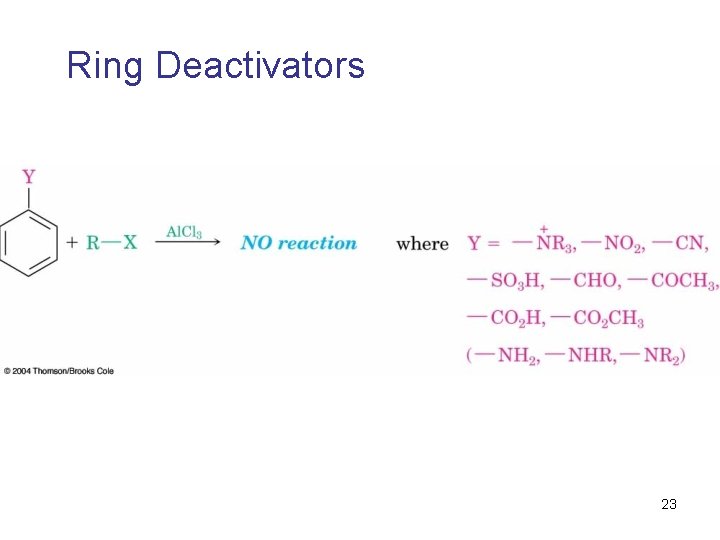
Ring Deactivators 23
Example 2: Friedel-Crafts Reaction • Diagram the mechanism for the electrophilic substitution of benzene by 2 -chloropentane: 24
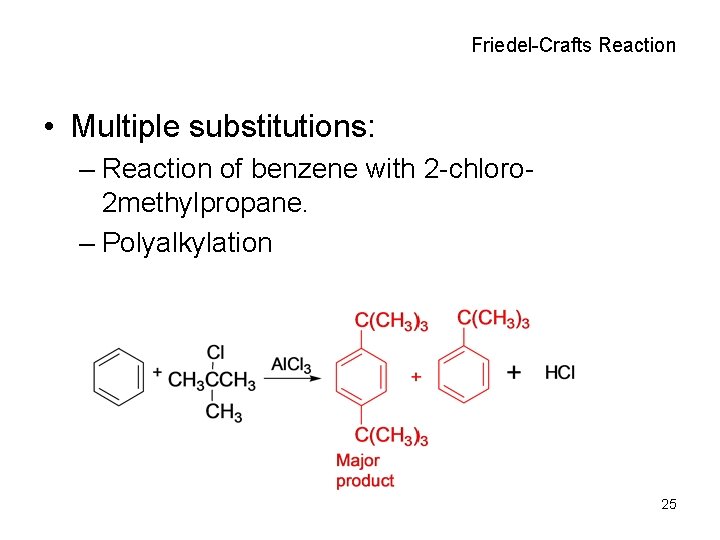
Friedel-Crafts Reaction • Multiple substitutions: – Reaction of benzene with 2 -chloro 2 methylpropane. – Polyalkylation 25
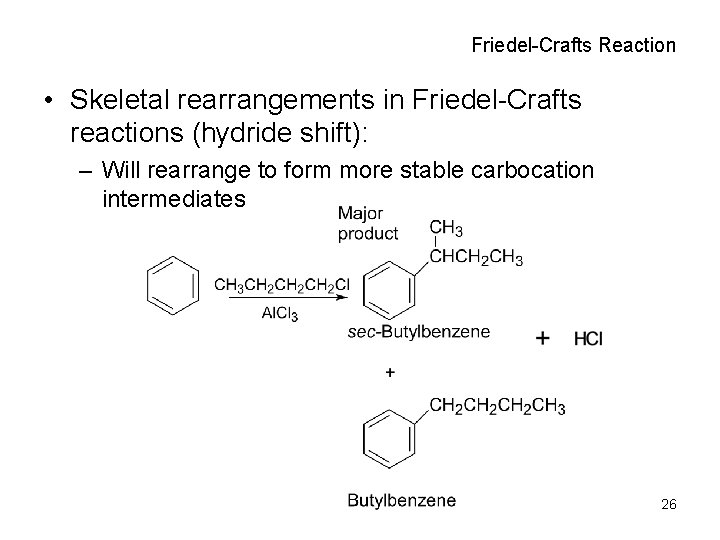
Friedel-Crafts Reaction • Skeletal rearrangements in Friedel-Crafts reactions (hydride shift): – Will rearrange to form more stable carbocation intermediates 26
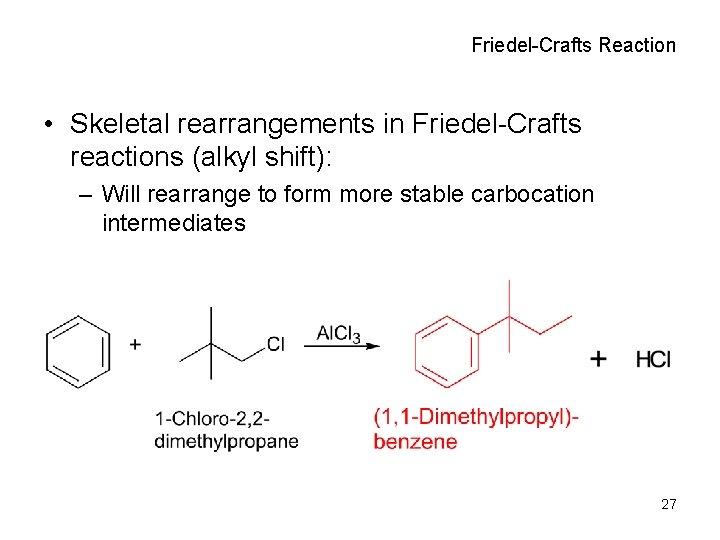
Friedel-Crafts Reaction • Skeletal rearrangements in Friedel-Crafts reactions (alkyl shift): – Will rearrange to form more stable carbocation intermediates 27

Example 3: • Which of the following alkyl halides would you expect to undergo Friedel-Crafts reaction without rearrangement? – Chloroethane – 2 -chlorobutane – 1 -chloropropane – 1 -chloro-2, 2 -dimethylpropane – Chlorocyclohexane 28

Friedel-Crafts Alkylation Summary • Only alkyl halides can be used!! • Will not occur on aromatic rings substituted by electron withdrawing substituents – Carbonyl and amino groups • Will have polyalkylation • Will have rearrangement to form more stable carbocation intermediate – Hydride shift or methyl shift • You need to know the mechanism!!! 29
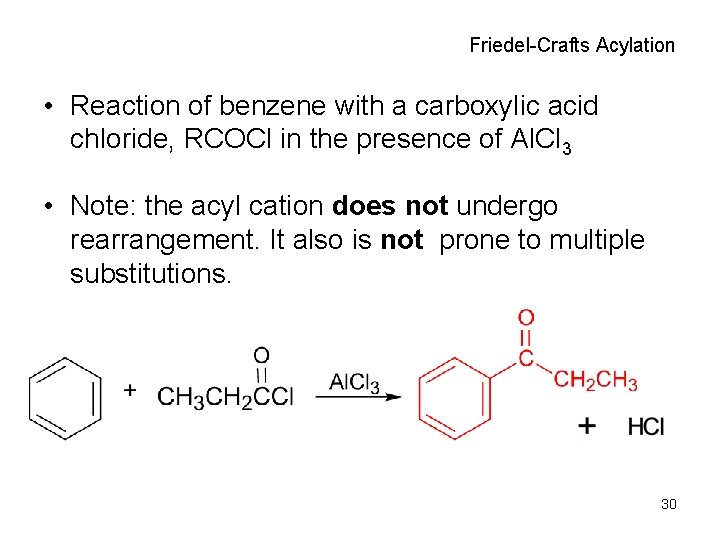
Friedel-Crafts Acylation • Reaction of benzene with a carboxylic acid chloride, RCOCl in the presence of Al. Cl 3 • Note: the acyl cation does not undergo rearrangement. It also is not prone to multiple substitutions. 30
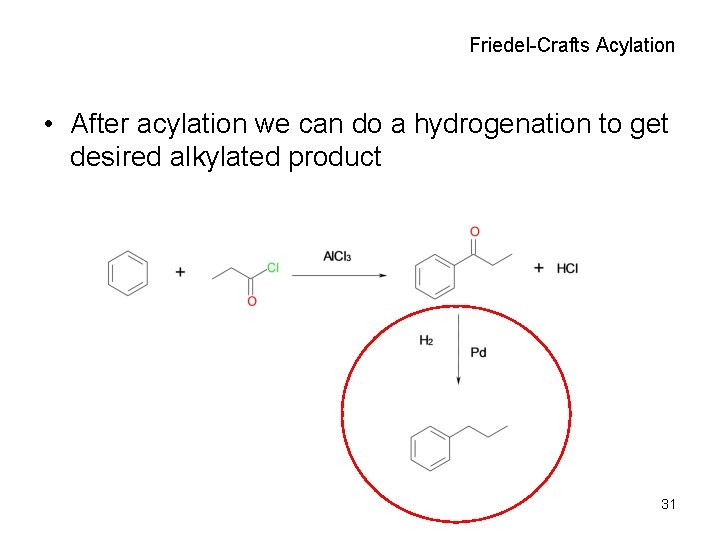
Friedel-Crafts Acylation • After acylation we can do a hydrogenation to get desired alkylated product 31
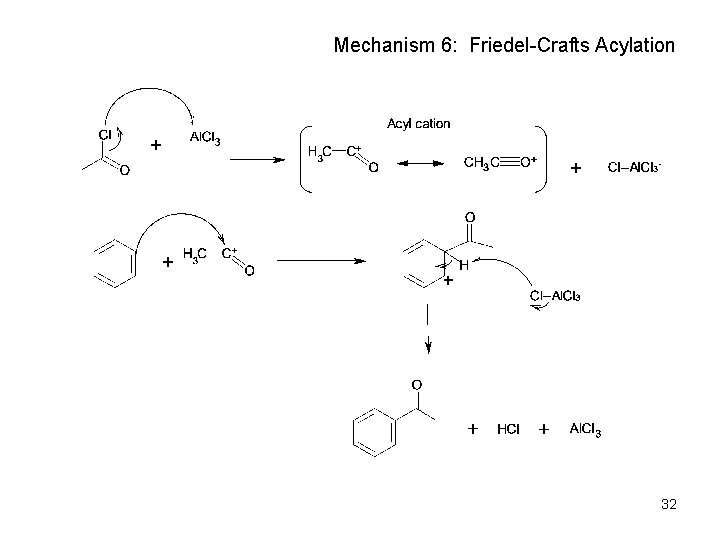
Mechanism 6: Friedel-Crafts Acylation 32
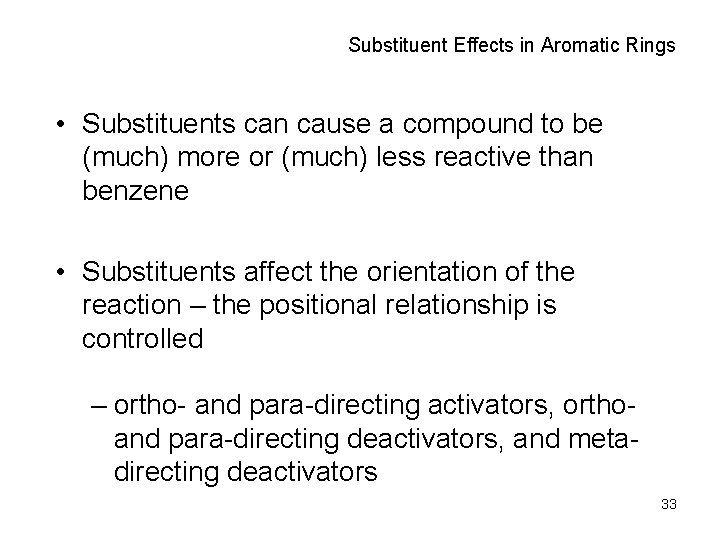
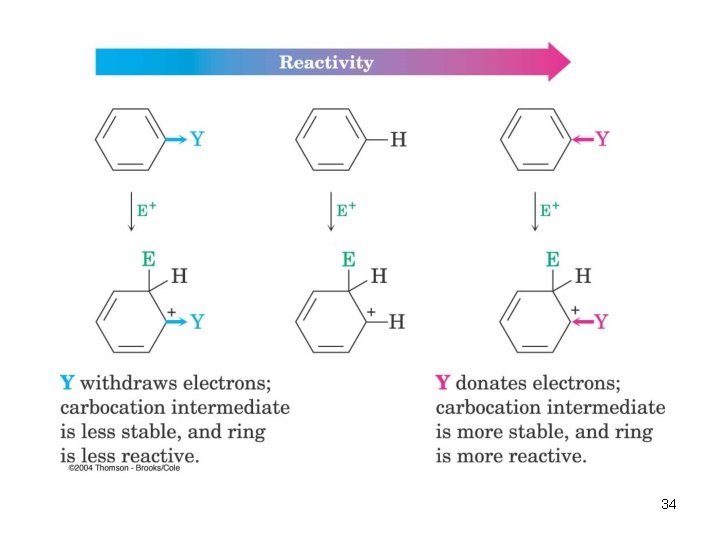
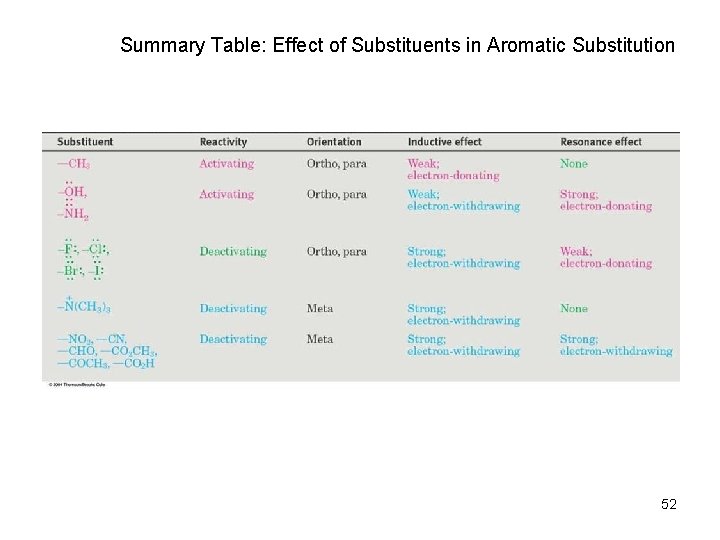
Substituent Effects in Aromatic Rings • Substituents can cause a compound to be (much) more or (much) less reactive than benzene • Substituents affect the orientation of the reaction – the positional relationship is controlled – ortho- and para-directing activators, orthoand para-directing deactivators, and metadirecting deactivators 33
34
35

Origins of Substituent Effects • An interplay of inductive effects and resonance effects • Inductive effect - withdrawal or donation of electrons through a s bond (comparative electronegativity) • Resonance effect - withdrawal or donation of electrons through a bond due to the overlap of a p orbital on the substituent with a p orbital on the aromatic ring 36

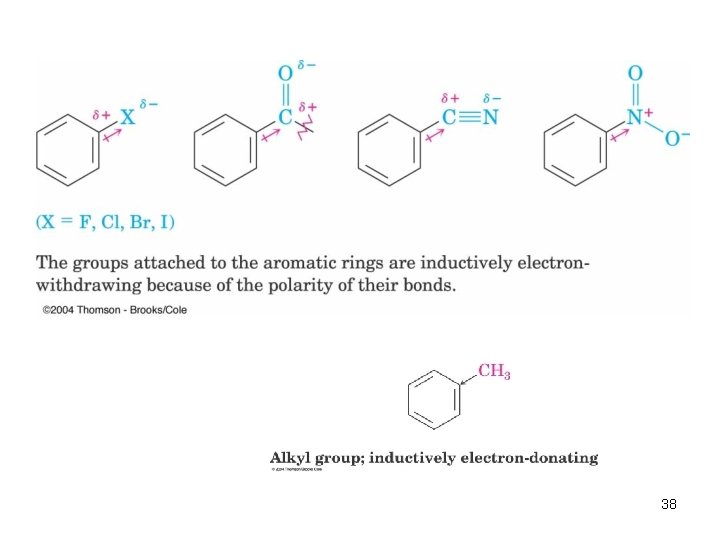
Inductive Effects • Controlled by electronegativity and the polarity of bonds in functional groups • Halogens, C=O, CN, and NO 2 withdraw electrons through s bond connected to ring • Alkyl groups donate electrons 37
38
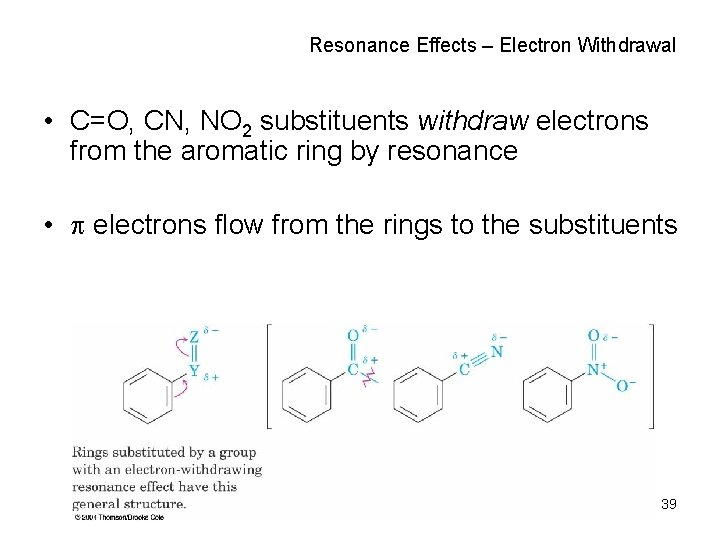
Resonance Effects – Electron Withdrawal • C=O, CN, NO 2 substituents withdraw electrons from the aromatic ring by resonance • electrons flow from the rings to the substituents 39
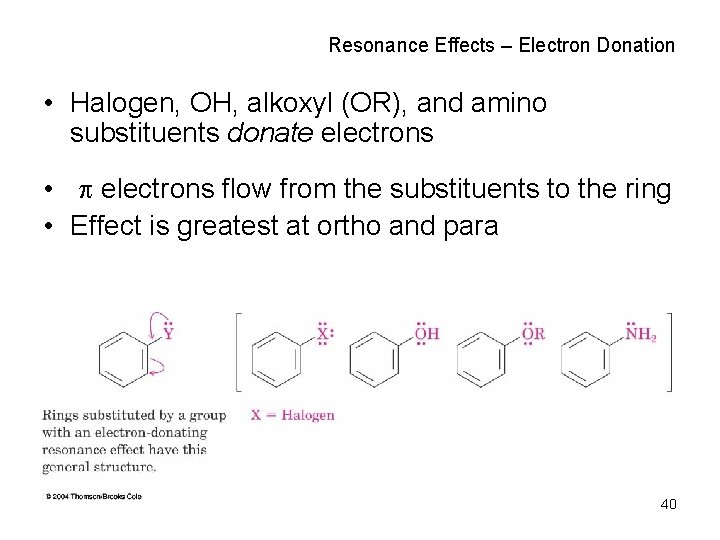
Resonance Effects – Electron Donation • Halogen, OH, alkoxyl (OR), and amino substituents donate electrons • electrons flow from the substituents to the ring • Effect is greatest at ortho and para 40

Contrasting Effects • Halogen, OH, OR, withdraw electrons inductively so that they deactivate the ring • Resonance interactions are generally weaker, affecting orientation • The strongest effects dominate 41
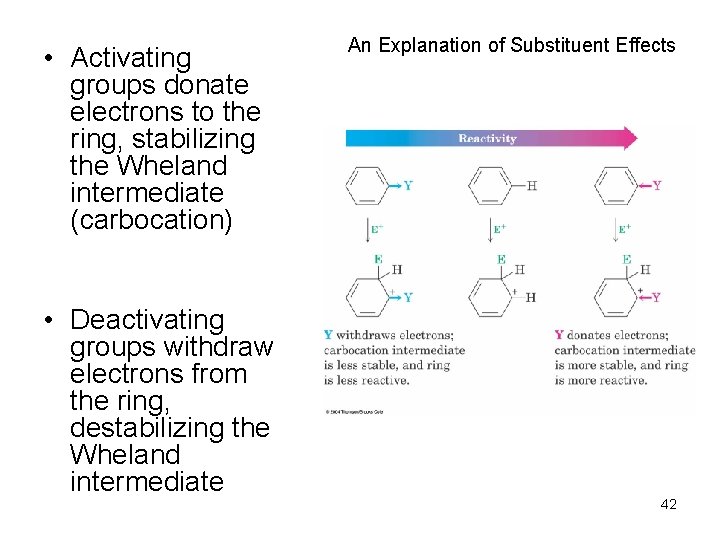
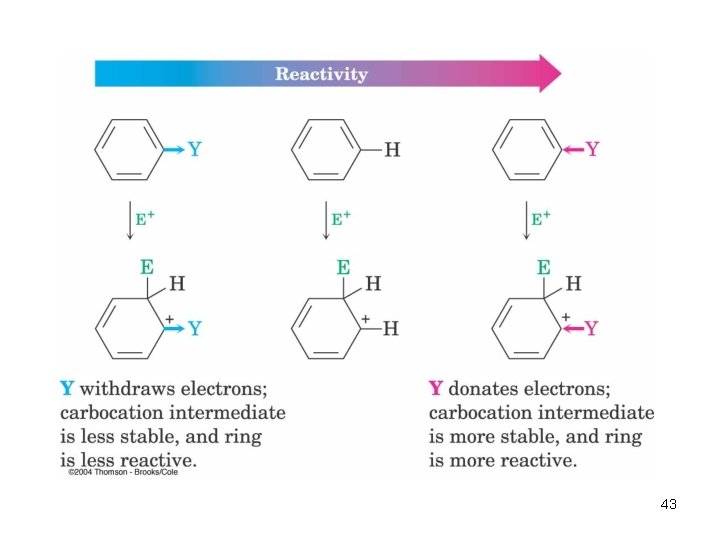
• Activating groups donate electrons to the ring, stabilizing the Wheland intermediate (carbocation) • Deactivating groups withdraw electrons from the ring, destabilizing the Wheland intermediate An Explanation of Substituent Effects 42
43

Ortho- and Para-Directing Activators: Alkyl Groups • Alkyl groups activate: direct further substitution to positions ortho and para to themselves • Alkyl group is most effective in the ortho and para positions 44
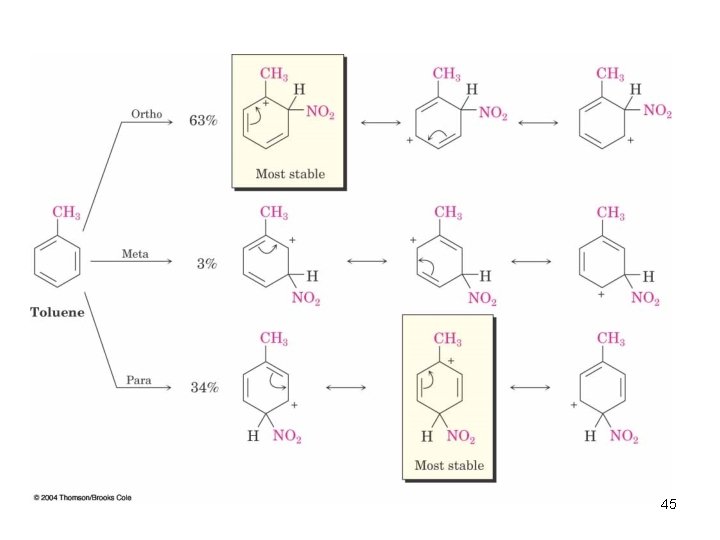
45

Ortho- and Para-Directing Activators: OH and NH 2 • Alkoxyl, and amino groups have a strong, electron-donating resonance effect • Most pronounced at the ortho and para positions 46
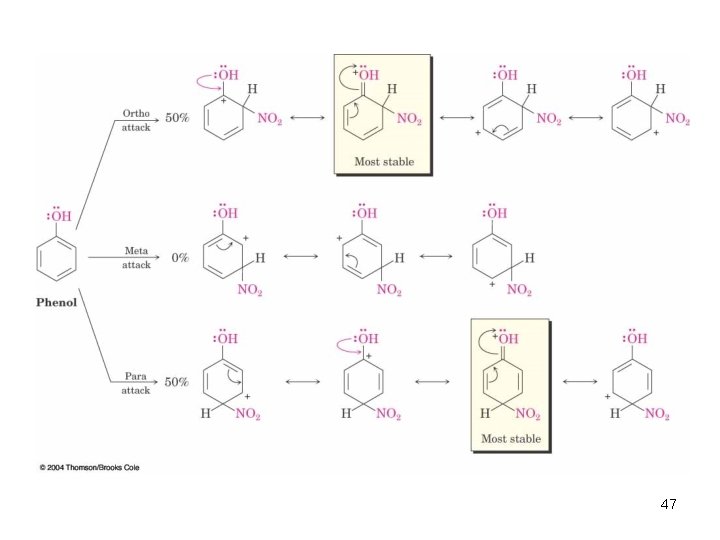
47
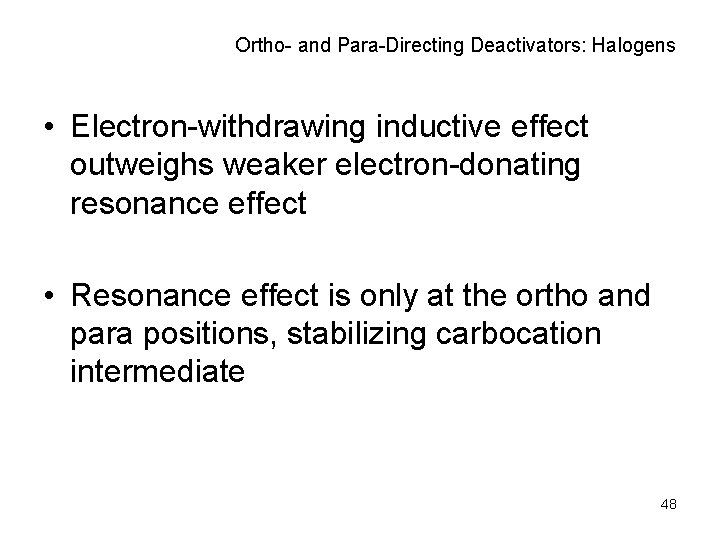
Ortho- and Para-Directing Deactivators: Halogens • Electron-withdrawing inductive effect outweighs weaker electron-donating resonance effect • Resonance effect is only at the ortho and para positions, stabilizing carbocation intermediate 48
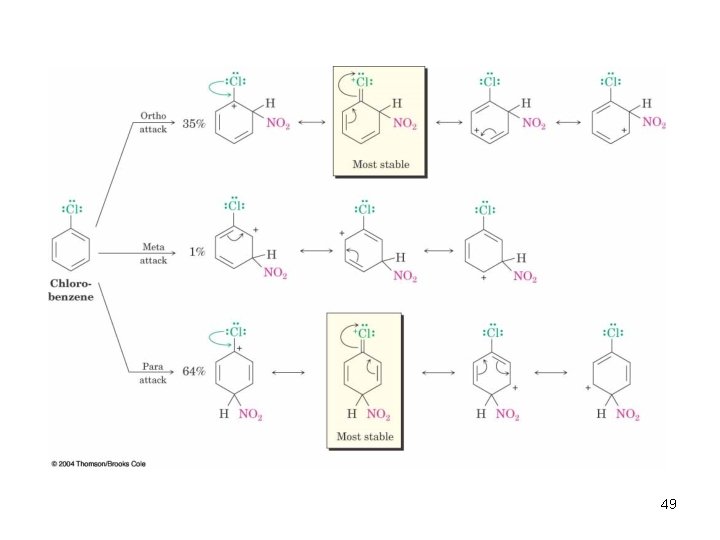
49

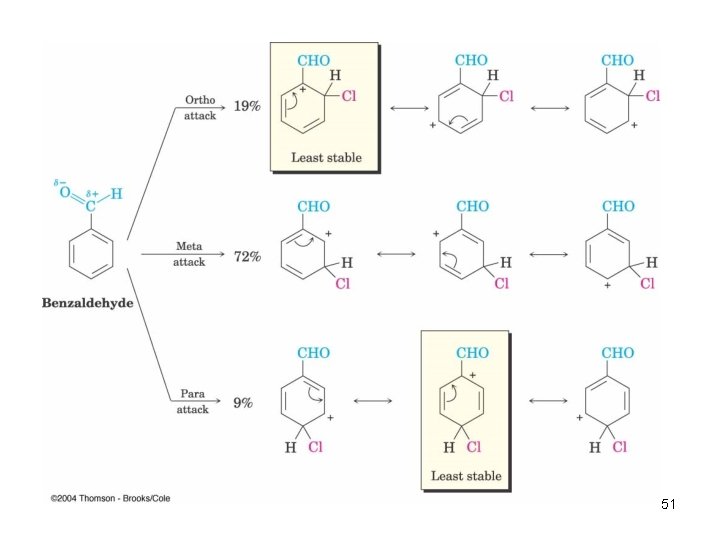
Meta-Directing Deactivators • Inductive and resonance effects reinforce each other • Ortho and para intermediates destabilized by deactivation from carbocation intermediate • Resonance cannot produce stabilization 50
51
Summary Table: Effect of Substituents in Aromatic Substitution 52
53

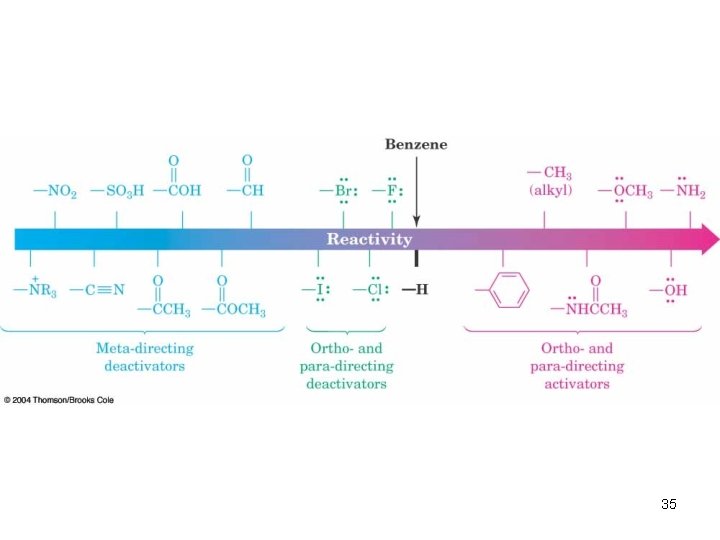
Is it ortho/para or meta directing? ? ? • All ortho- and para- directors have a lone pair of electrons on the atom directly attached to the ring (with the exception of alkyl, aryl, and CH=CHR groups). • All meta- directors have a positive charge or a partial positive charge on the atom attached to the ring. 54

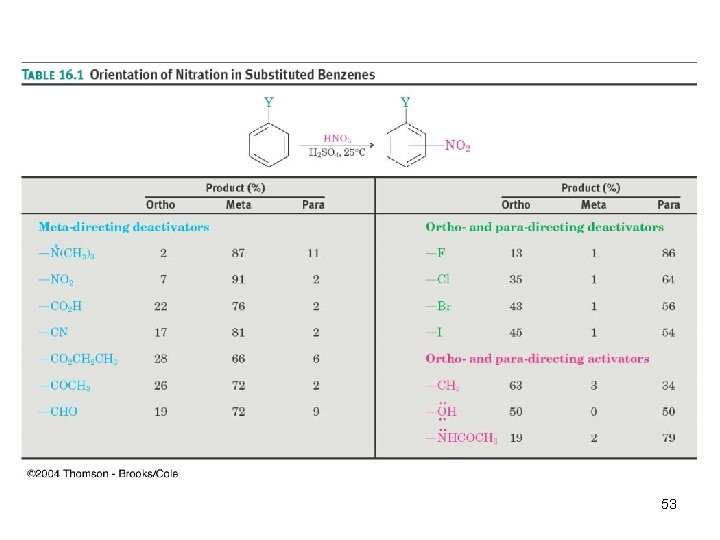
In Summary: • All activating substituents are ortho/para directors • The weakly deactivating halogens are ortho/para directors • All other deactivating substituents are meta directors 55
Example 4: 56

Example 5: What product(s) would result from the nitration of each of the following compounds? • • • propylbenzenesulfonic acid iodobenzene benzaldehyde cyclohexylbenzene benzonitrile 57
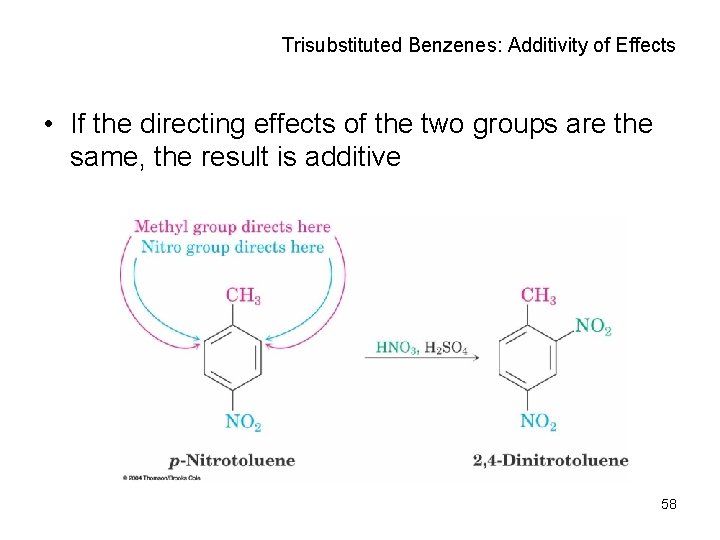
Trisubstituted Benzenes: Additivity of Effects • If the directing effects of the two groups are the same, the result is additive 58
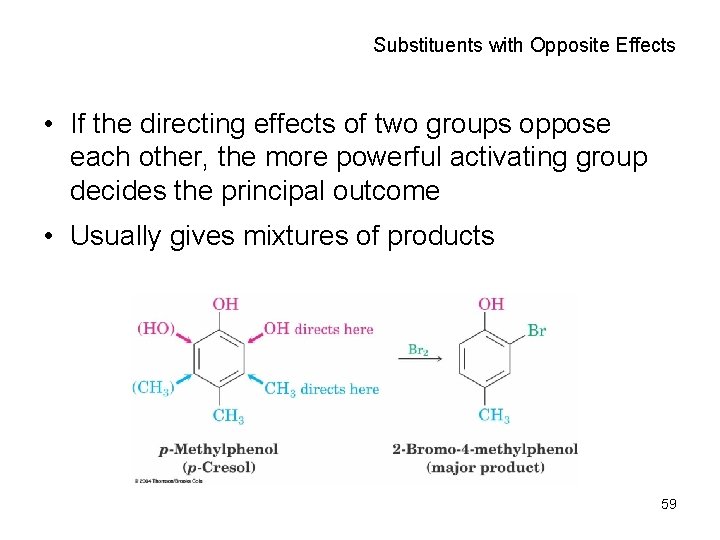
Substituents with Opposite Effects • If the directing effects of two groups oppose each other, the more powerful activating group decides the principal outcome • Usually gives mixtures of products 59

Meta-Disubstituted Compounds Are Unreactive • The reaction site is too hindered • To make aromatic rings with three adjacent substituents, it is best to start with an ortho-disubstituted compound 60
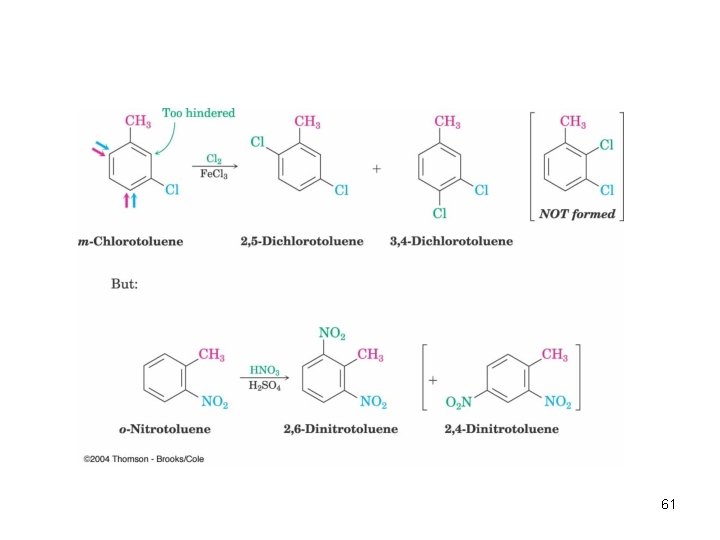
61
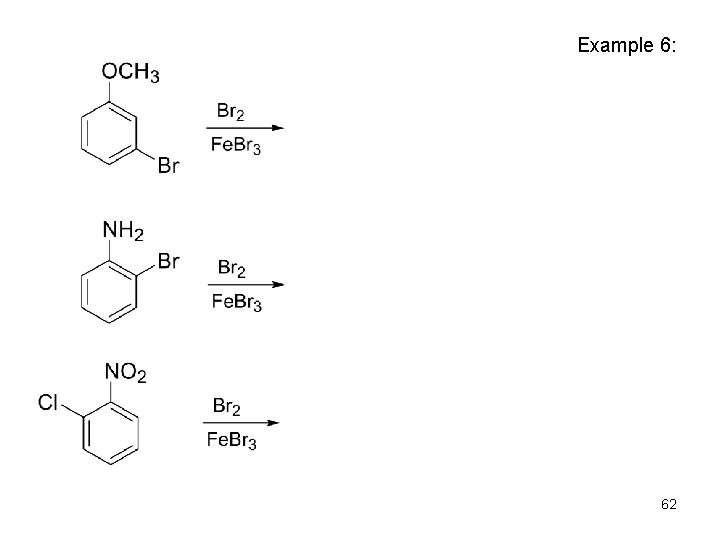
Example 6: 62
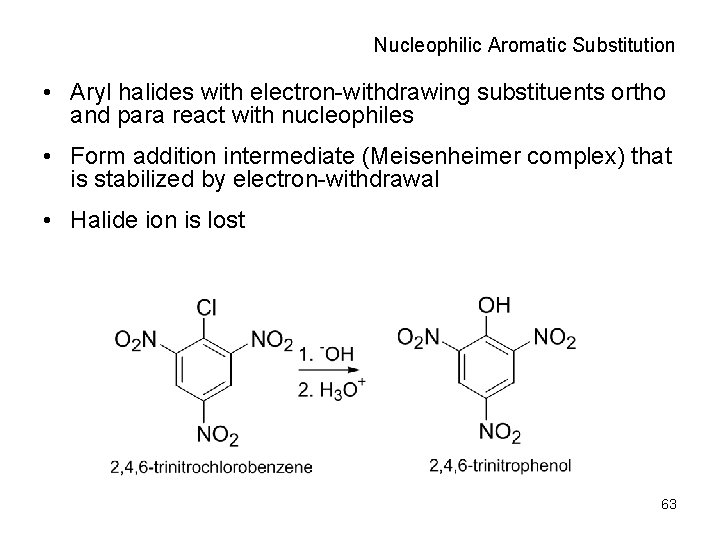
Nucleophilic Aromatic Substitution • Aryl halides with electron-withdrawing substituents ortho and para react with nucleophiles • Form addition intermediate (Meisenheimer complex) that is stabilized by electron-withdrawal • Halide ion is lost 63
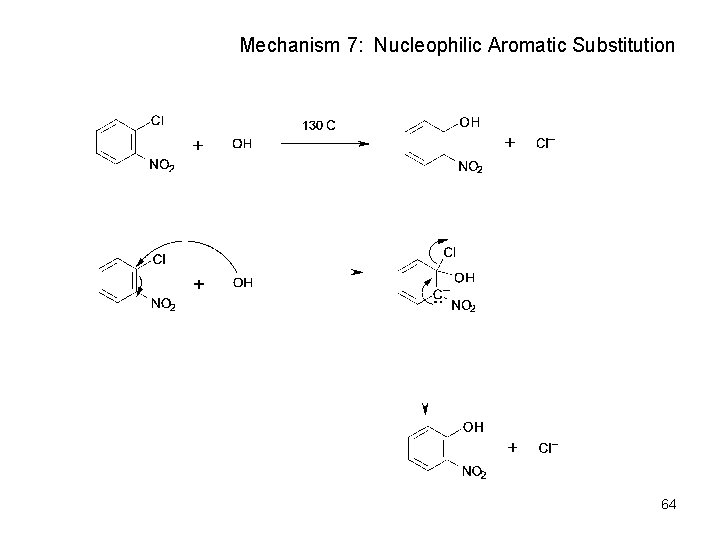
Mechanism 7: Nucleophilic Aromatic Substitution 64
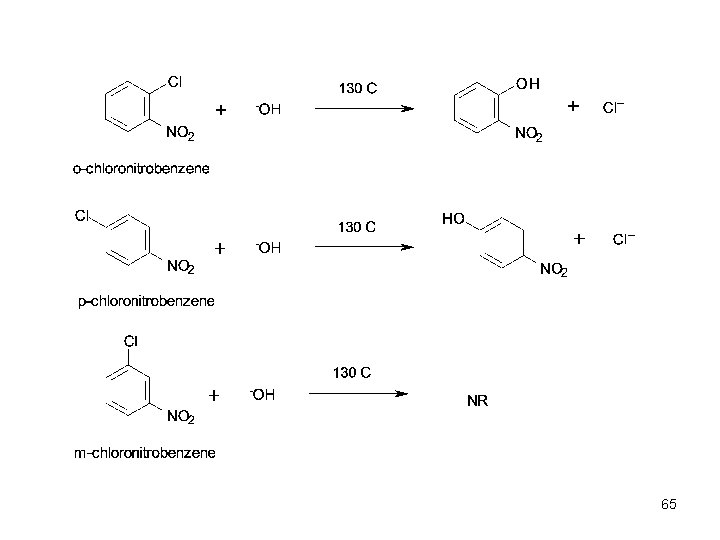
65
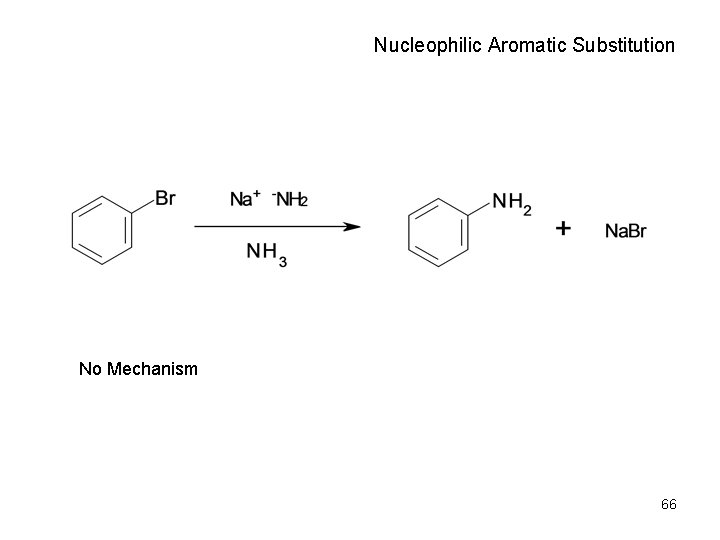
Nucleophilic Aromatic Substitution No Mechanism 66

Electrophilic and Nucleophilic Substitution • Electrophilic Sub – Favored by electron donating substituents • Stabilize carbocation intermediate • Nucleophilic Sub – Favored by electron withdrawing substituents • Stabilize carbanion intermediate 67
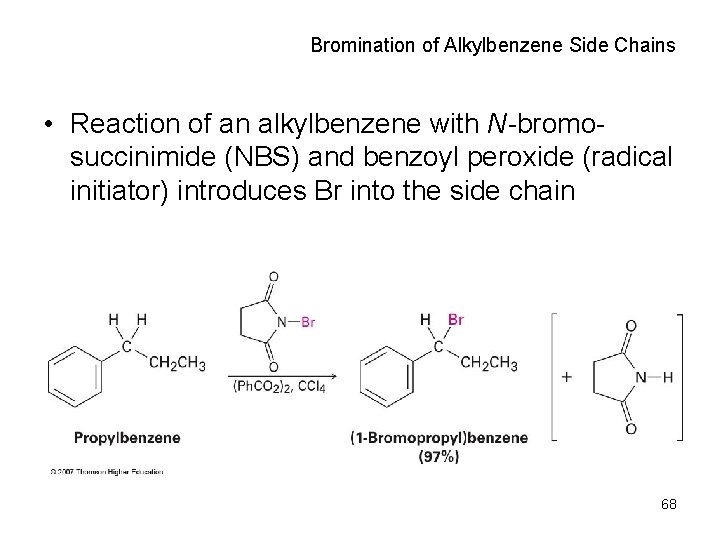
Bromination of Alkylbenzene Side Chains • Reaction of an alkylbenzene with N-bromosuccinimide (NBS) and benzoyl peroxide (radical initiator) introduces Br into the side chain 68
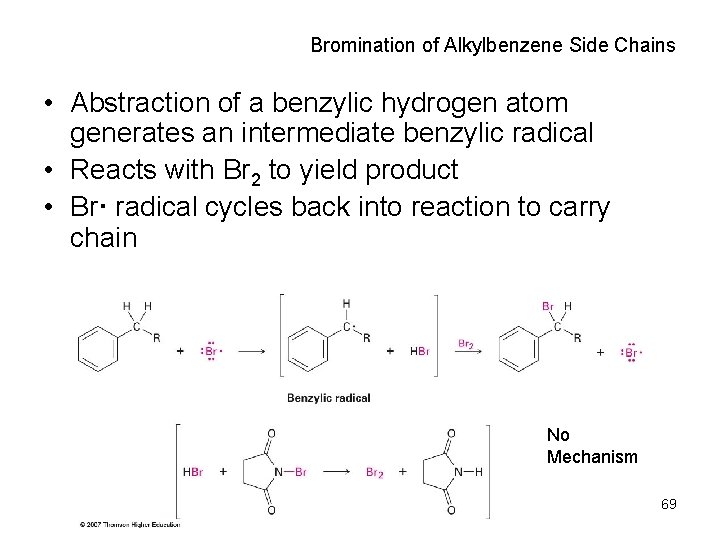
Bromination of Alkylbenzene Side Chains • Abstraction of a benzylic hydrogen atom generates an intermediate benzylic radical • Reacts with Br 2 to yield product • Br· radical cycles back into reaction to carry chain No Mechanism 69
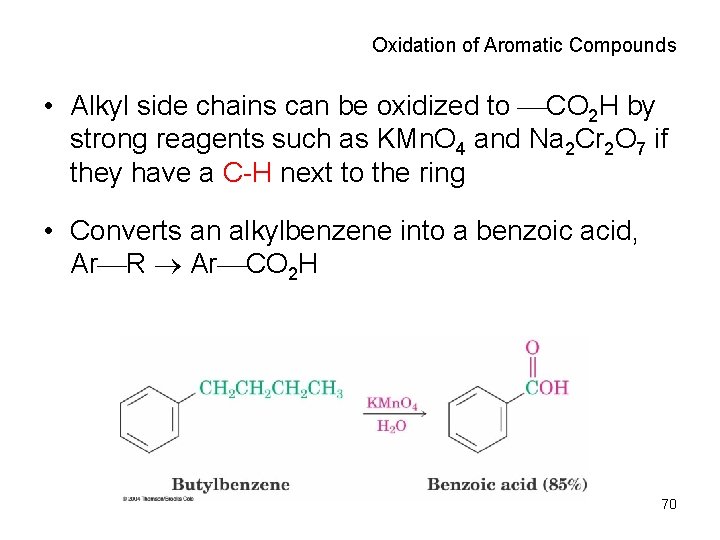
Oxidation of Aromatic Compounds • Alkyl side chains can be oxidized to CO 2 H by strong reagents such as KMn. O 4 and Na 2 Cr 2 O 7 if they have a C-H next to the ring • Converts an alkylbenzene into a benzoic acid, Ar R Ar CO 2 H 70
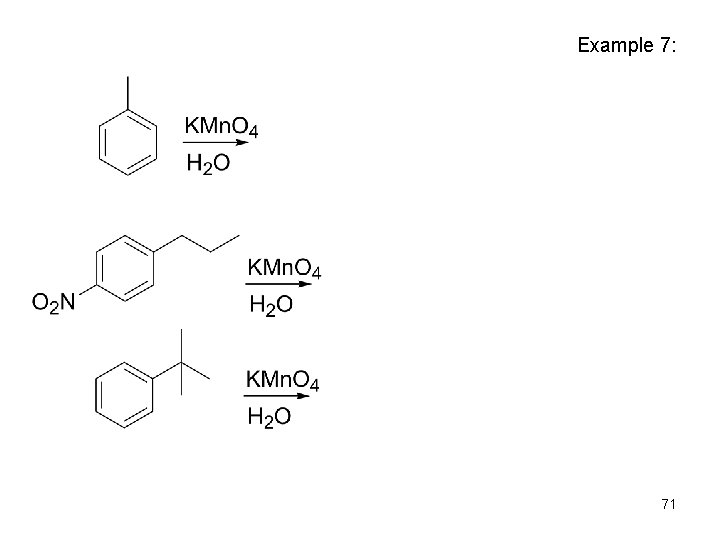
Example 7: 71
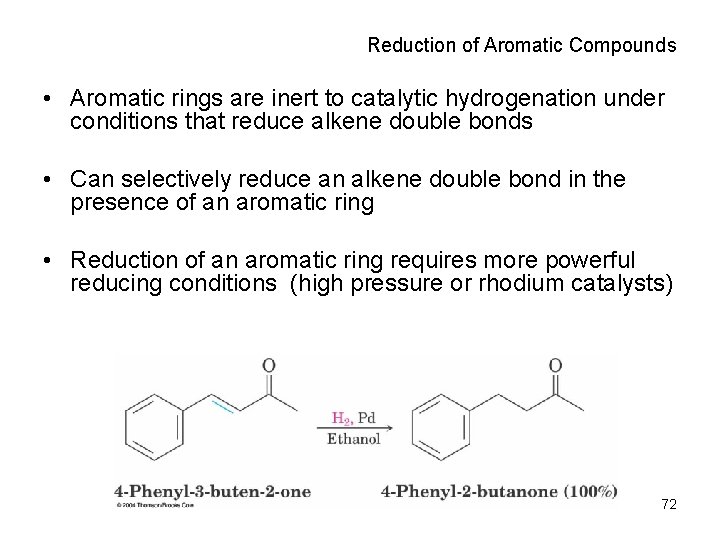
Reduction of Aromatic Compounds • Aromatic rings are inert to catalytic hydrogenation under conditions that reduce alkene double bonds • Can selectively reduce an alkene double bond in the presence of an aromatic ring • Reduction of an aromatic ring requires more powerful reducing conditions (high pressure or rhodium catalysts) 72
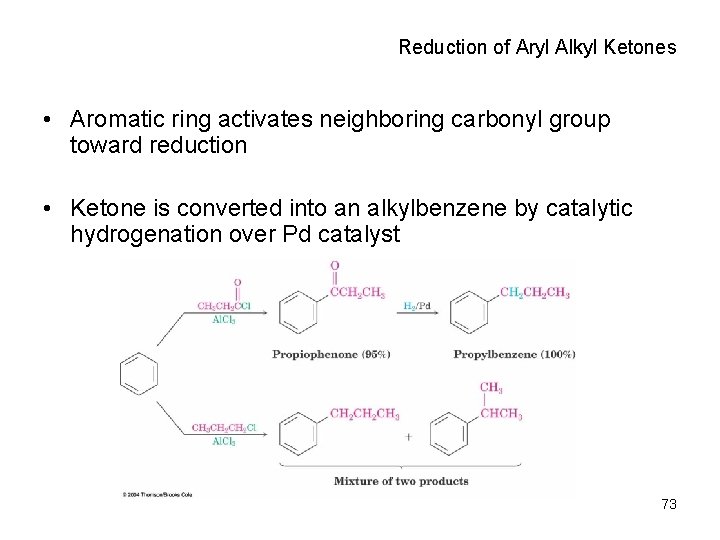
Reduction of Aryl Alkyl Ketones • Aromatic ring activates neighboring carbonyl group toward reduction • Ketone is converted into an alkylbenzene by catalytic hydrogenation over Pd catalyst 73
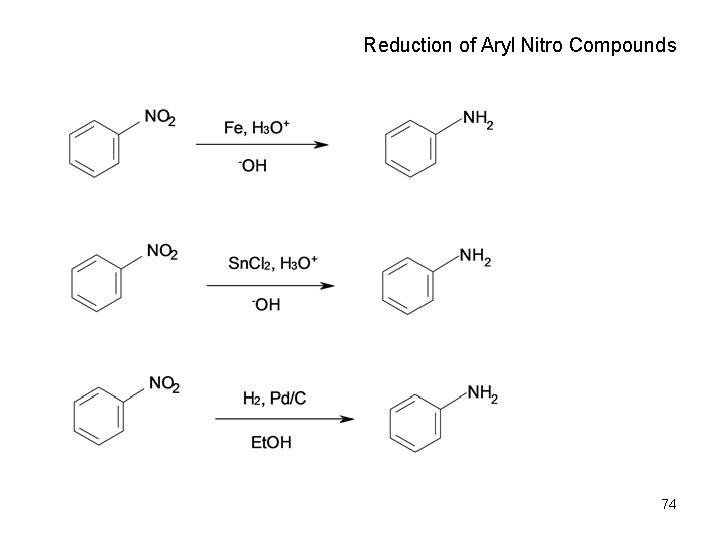
Reduction of Aryl Nitro Compounds 74
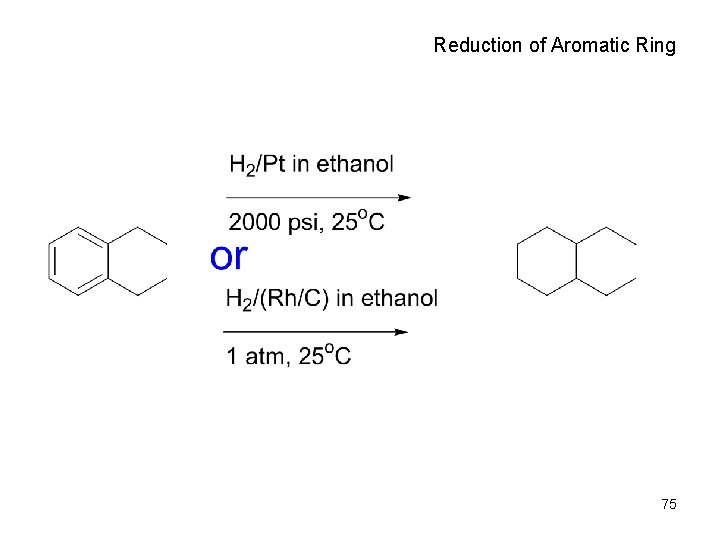
Reduction of Aromatic Ring 75

Synthesis Strategies • These syntheses require planning and consideration of alternative routes • It’s important to pay attention to the order in which substituents are placed on the ring – meta or or ortho/para directing • When should an added substituent be modified? 76

Example 8: Synthesize the following 1. m-bromobenzenesulfonic acid from benzene 2. p-bromobenzenesulfonic acid from benzene 3. p-propylbenzenesulfonic acid from benzene 4. 2 -bromo-4 -ethylphenol from benzene 77
- Slides: 77