Ch 15 MATTER PURE SUBSTANCES Pure substance blend
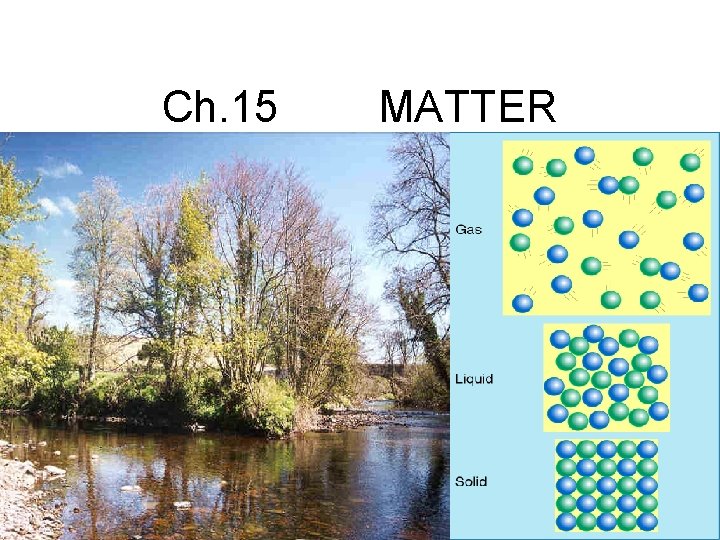
Ch. 15 MATTER
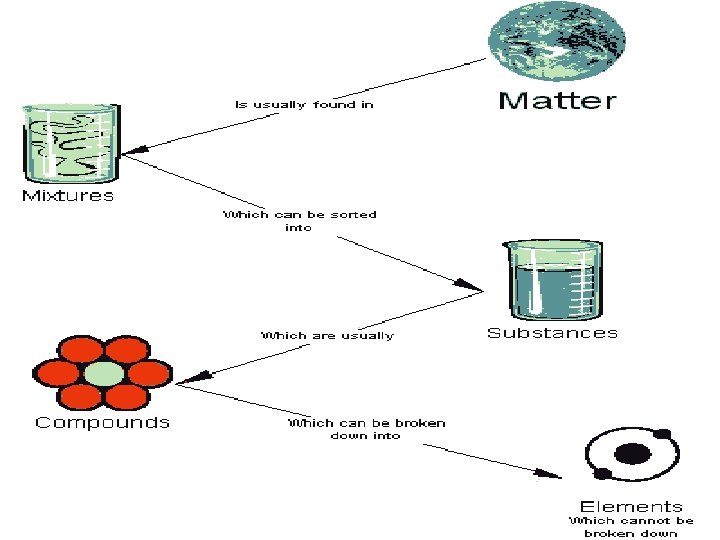

PURE SUBSTANCES • Pure substance blend together to make mixtures – Ex. elements/compounds/molecules – CHEMICALLY BONDED • Both have chemical formulas – C 16 H 10 N 2 O 2 BLUE JEANS-INDIGO – 3 C 16 H 10 N 2 O 2 3 is the # of molecules (Coefficient)

Heterogeneous Mixtures • Particles in suspension are large, and settle out – Ex. )Pulpy orange juice • Particles in a colloid are smaller and do not settle out-Tyndall Effect-how light scatters from the particles – Ex. ) Gelatin-tiny particles of proteins mixed w/ water (egg white, paint, blood) • Liquid-liquid mixtures carefully unmixed – Oil/ Water, Fat/Oil • Immiscible can mix in emulsions – Mayo-oil in vinegar, egg yolks keeps them together

Homogeneous Mixture • Uniform, even under a microscope – Salt Water- all clear! • Solutions=homogeneous – Solution=solute +solvent • Solution: Salt Water • Solute=Salt • Solvent= Water (the universal solvent) • Miscible liquids form solutions – Distillation/Chromotography seperation

• Kinetic Theory 1. All matter is made of atoms 2. The particles are always in motion- higher temp, the faster they move 3. At the same temp. , larger particles move slower Temperature=Average Kinetic Energy 0= Freeze 100=Boil
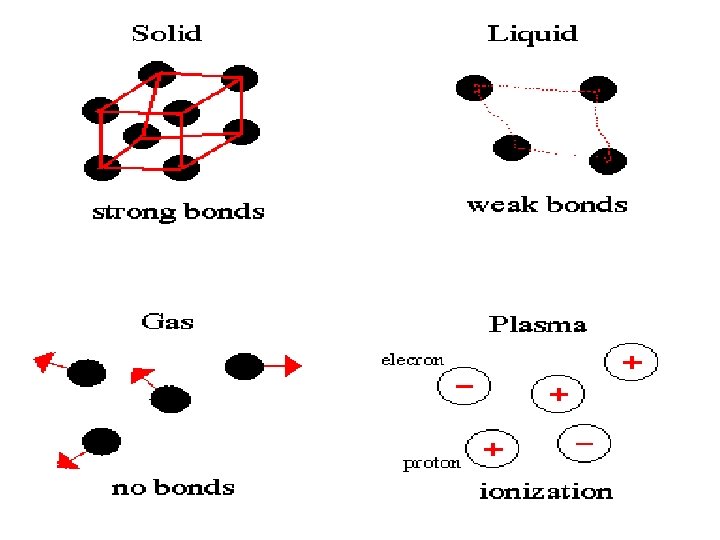
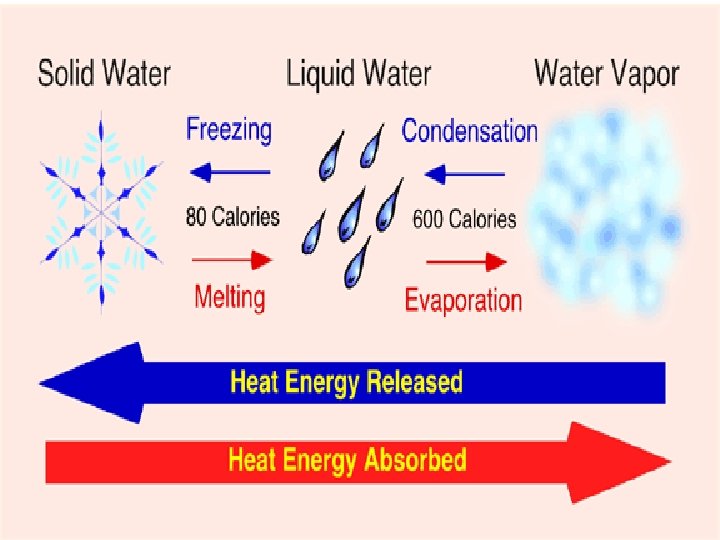

• Chemical Properties • Reactivity – Chemical Changes • Change in composition • Permanent • Physical Properties • • Melting/boiling Density D=m/v Buoyancy color/odor/shape – Physical Changes • Not chemical • Dissolving • Phase changes are physical
- Slides: 9