Ch 15 and 6 Naming and Writing Formulas





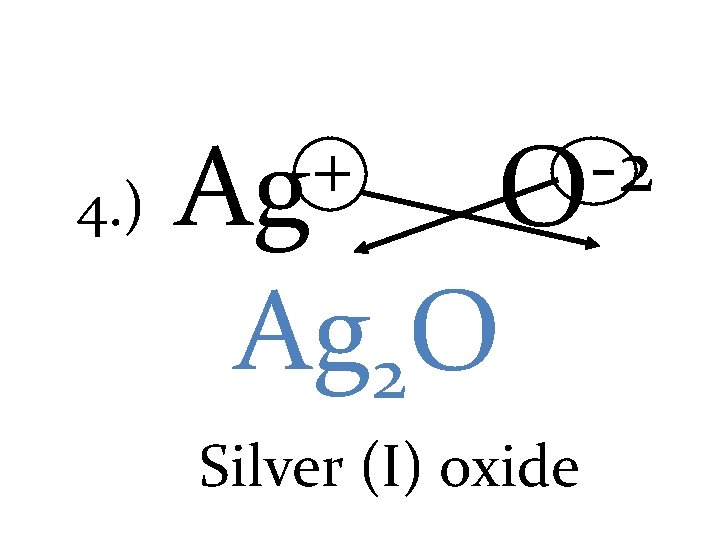

- Slides: 19

Ch. 15 and 6 Naming and Writing Formulas for Ionic Compounds

Writing Formulas for Binary Ionic Compounds Ch. 6 A. Formula Unit: the lowest whole-number ratio of ions in an ionic compound

Writing Formulas for Binary Ionic Compounds B. Rules: 1. Write symbol and charge for both cation and anion. 2. Crisscross the numbers for the charges and write as subscripts. 3. CHECK!! The formula must be in the lowest possible ratio of ions (this means reduce). The cation is always written first!

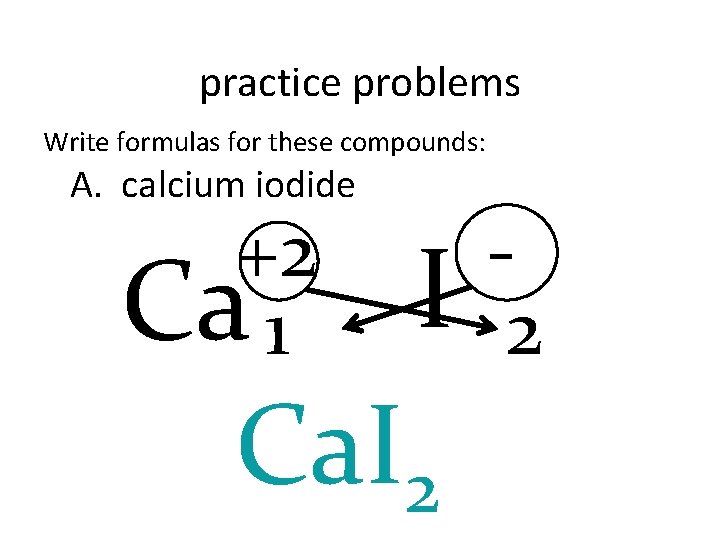
practice problems Write formulas for these compounds: A. calcium iodide +2 Ca 1 I Ca. I 2 2

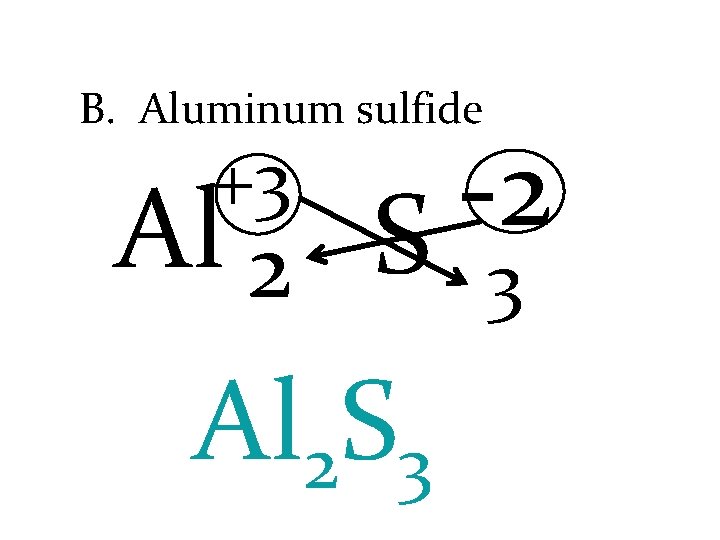
B. Aluminum sulfide +3 Al 2 -2 S Al 2 S 3 3

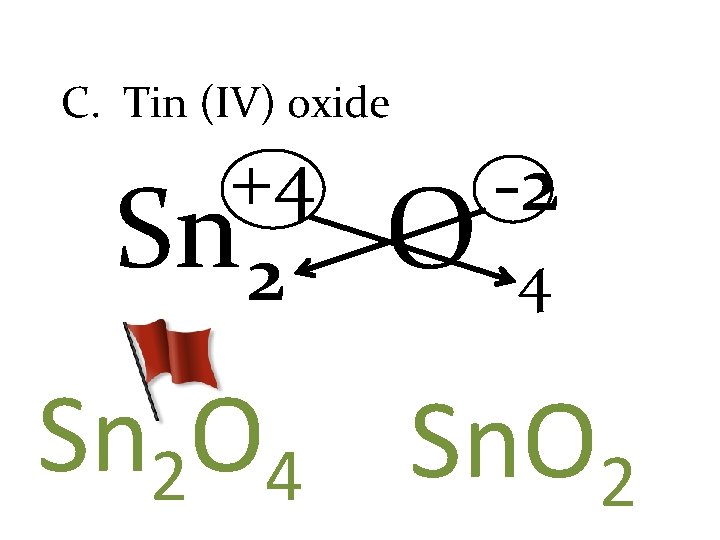
C. Tin (IV) oxide +4 Sn 2 O -2 4 Sn 2 O 4 Sn. O 2

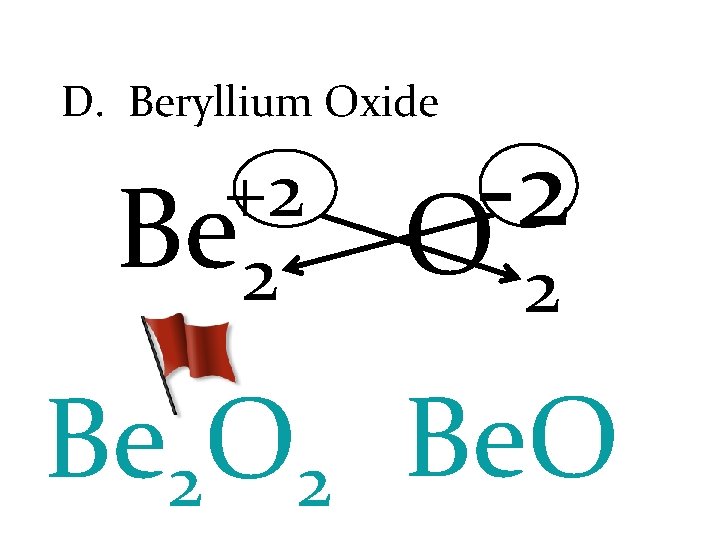
D. Beryllium Oxide +2 Be 2 -2 O 2 Be 2 O 2 Be. O

Naming Binary Ionic Compounds 1. ) Name the cation. Use Roman Numerals if Transition Metal. (Middle Name, a roman numeral) 2. ) Name the anion. Drop their last names ending, put –ide in its place Na. Cl Sodium Ion Chlorine Ion Sodium Chloride

Naming Binary Ionic Compounds that contain a transition metal • Use Roman numerals (middle name) to show the charge of the ion. Many of these elements can form more than one ion: Cr 2+ Chromium II or Cr 3+ Chromium III Cu+ Copper I or Cu 2+ Copper II Mn 2+ Manganese II or Mn 3+ Manganese III Fe 2+ Iron II or Fe 3+ Iron III Co 2+ Cobalt II or Co 3+ Cobalt III Sn 2+ Tin II or Sn 4+ Tin IV Pb 2+ Lead II or Pb 4+ Lead IV Zn 1+ Zinc I or Zn 2+ Zinc II Ni 2+ Nickel II or Ni 3+ Nickel III

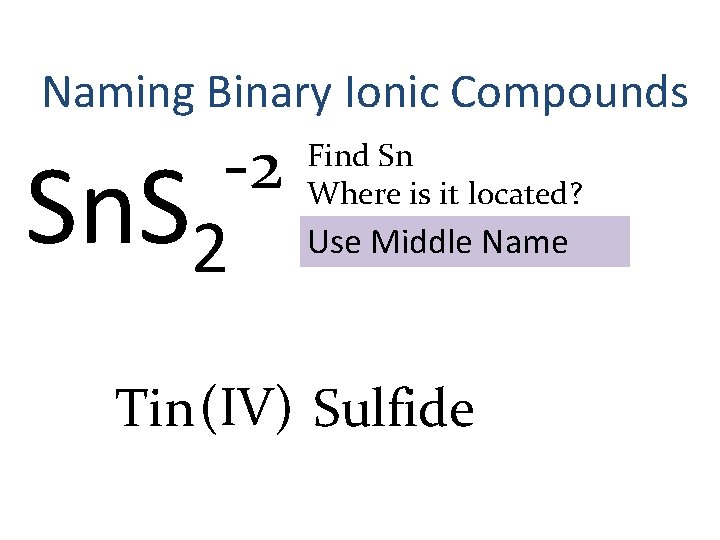
Naming Binary Ionic Compounds -2 Sn. S 2 Find Sn Where is it located? Use Middle Name Tin (IV) Sulfide

practice problems A. ) KCl Potassium chloride B. ) Cu. Br 2 Copper (II) bromide C. ) Al. F 3 Aluminum fluoride D. ) Mg. O Magnesium oxide E. ) Mn. I 2 F. ) Zn. S Manganese (II) iodide zinc (II) sulfide

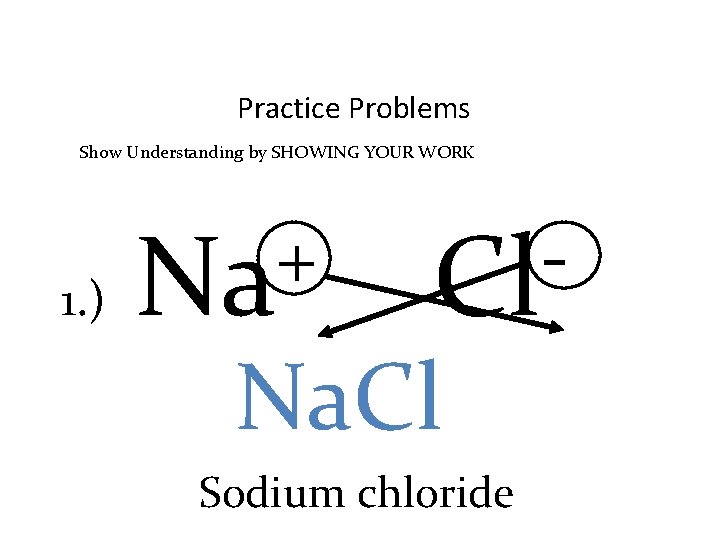
Practice Problems Show Understanding by SHOWING YOUR WORK 1. ) + Na Cl Na. Cl Sodium chloride

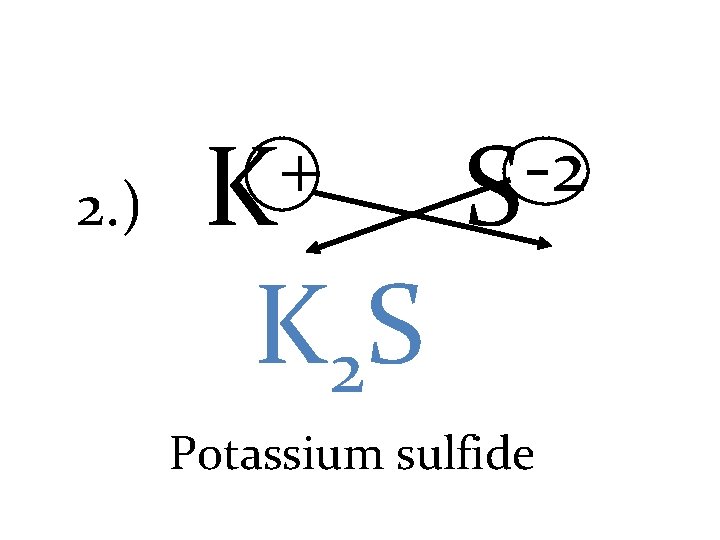
2. ) + K K 2 S -2 S Potassium sulfide

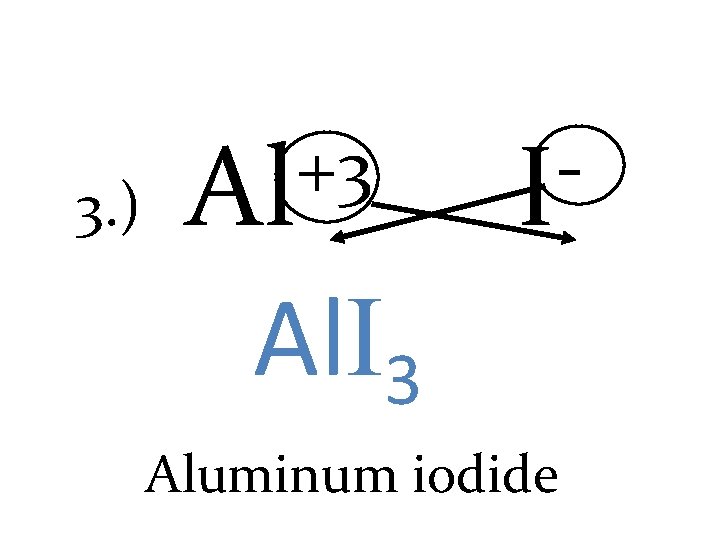
3. ) +3 Al I Al. I 3 Aluminum iodide
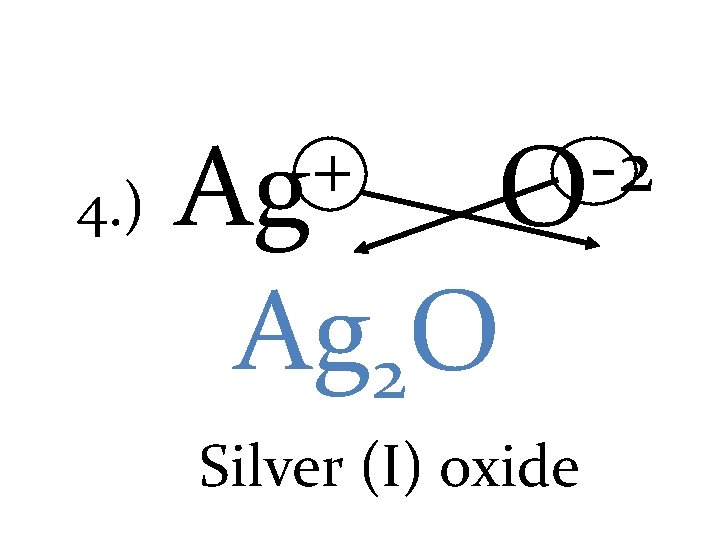
4. ) + Ag -2 O Ag 2 O Silver (I) oxide

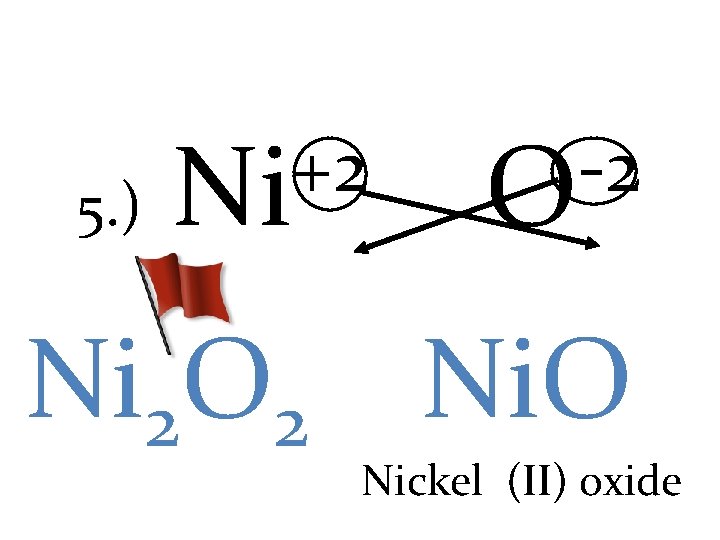
5. ) +2 Ni -2 O Ni 2 O 2 Ni. O Nickel (II) oxide

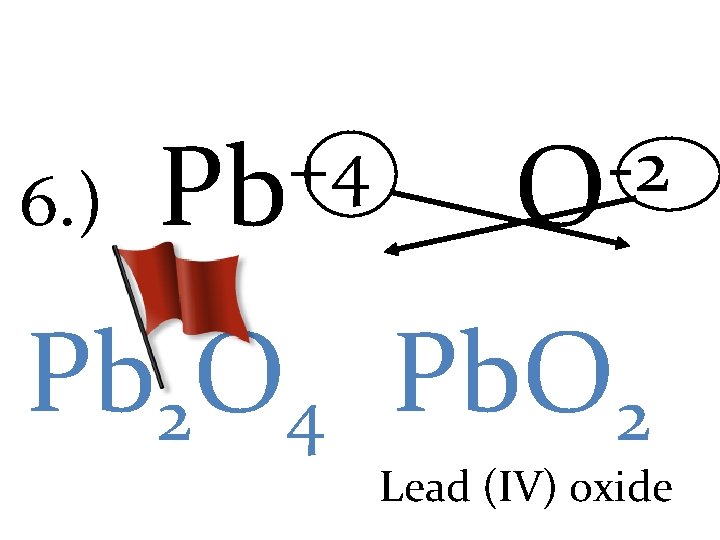
+4 6. ) Pb -2 O Pb 2 O 4 Pb. O 2 Lead (IV) oxide

Ionic Review Write formulas for these binary ionic compounds a. Barium Sulfide Ba. S Sn. Cl 4 b. Tin (IV) Chloride Mg 3 N 2 c. Magnesium Nitride d. Sodium Oxide Na 2 O

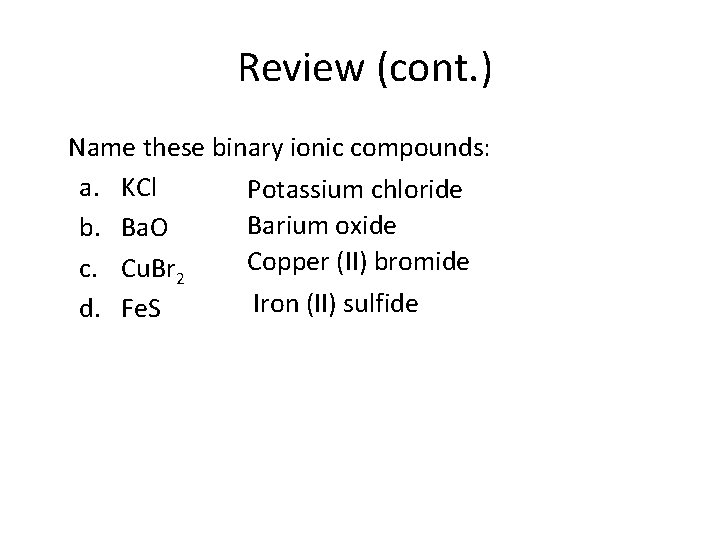
Review (cont. ) Name these binary ionic compounds: a. KCl Potassium chloride Barium oxide b. Ba. O Copper (II) bromide c. Cu. Br 2 Iron (II) sulfide d. Fe. S