Ch 14 Surface Hardening and Modification of Metals


- Slides: 21

Ch. 14 Surface Hardening and Modification of Metals /MS 371/ Structure and Properties of Engineering Alloys

Introduction • Surface Treatment – – Thermochemical treatments to Also called hardening May or may not require quenching Interior to remain the surface part: C, N • Reason for Surface Treatment – – – Increase resistance Increase surface strength for carrying (crush resistance) Induce suitable residual and compressive Improve fatigue life Impact resistance /MS 371/ Structure and Properties of Engineering Alloys

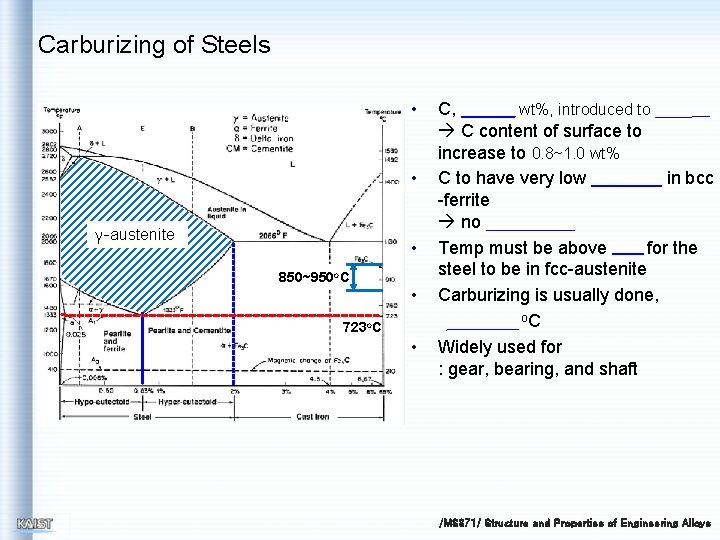
Carburizing of Steels • • γ-austenite • 850~950 o. C • 723 o. C • C, wt%, introduced to C content of surface to increase to 0. 8~1. 0 wt% C to have very low in bcc -ferrite no Temp must be above for the steel to be in fcc-austenite Carburizing is usually done, o. C Widely used for : gear, bearing, and shaft /MS 371/ Structure and Properties of Engineering Alloys

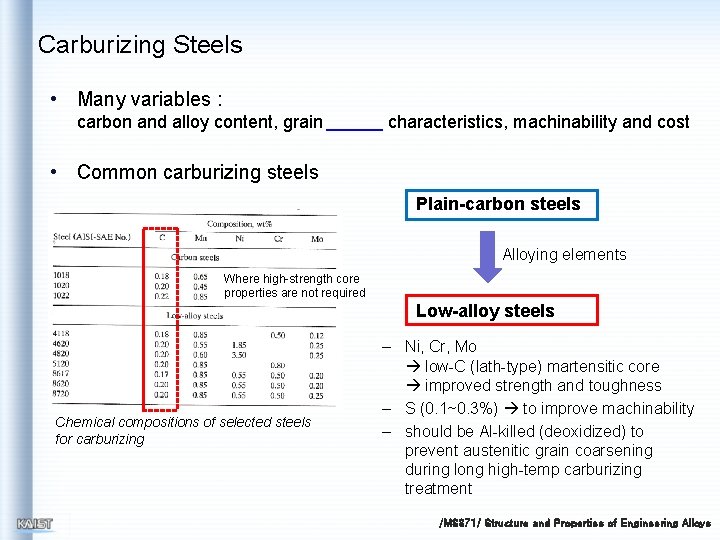
Carburizing Steels • Many variables : carbon and alloy content, grain characteristics, machinability and cost • Common carburizing steels Plain-carbon steels Alloying elements Where high-strength core properties are not required Low-alloy steels Chemical compositions of selected steels for carburizing – Ni, Cr, Mo low-C (lath-type) martensitic core improved strength and toughness – S (0. 1~0. 3%) to improve machinability – should be Al-killed (deoxidized) to prevent austenitic grain coarsening during long high-temp carburizing treatment /MS 371/ Structure and Properties of Engineering Alloys

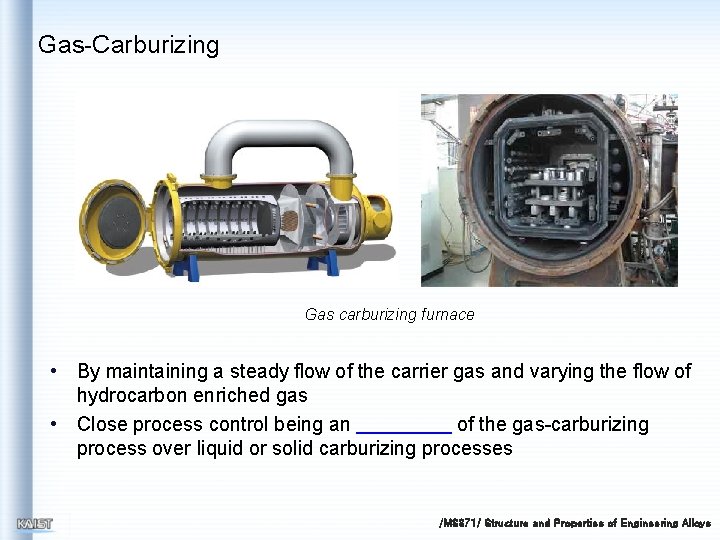
Gas-Carburizing Gas carburizing furnace • By maintaining a steady flow of the carrier gas and varying the flow of hydrocarbon enriched gas • Close process control being an of the gas-carburizing process over liquid or solid carburizing processes /MS 371/ Structure and Properties of Engineering Alloys

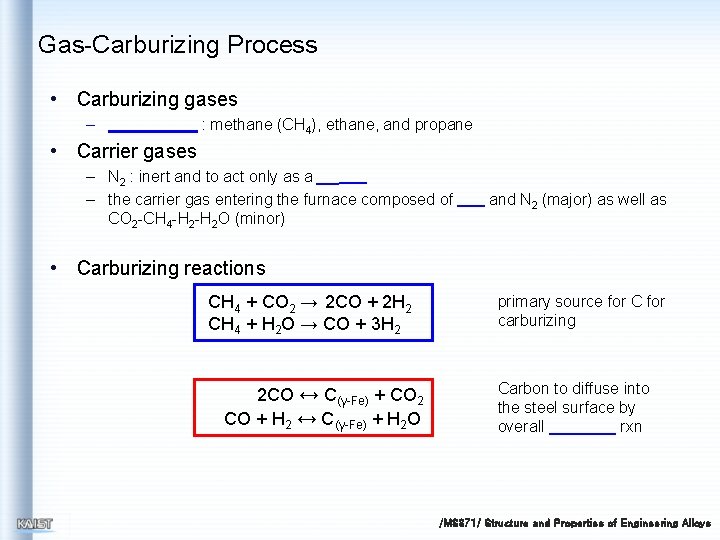
Gas-Carburizing Process • Carburizing gases – : methane (CH 4), ethane, and propane • Carrier gases – N 2 : inert and to act only as a – the carrier gas entering the furnace composed of CO 2 -CH 4 -H 2 O (minor) and N 2 (major) as well as • Carburizing reactions CH 4 + CO 2 → 2 CO + 2 H 2 CH 4 + H 2 O → CO + 3 H 2 2 CO ↔ C(γ-Fe) + CO 2 CO + H 2 ↔ C(γ-Fe) + H 2 O primary source for C for carburizing Carbon to diffuse into the steel surface by overall rxn /MS 371/ Structure and Properties of Engineering Alloys

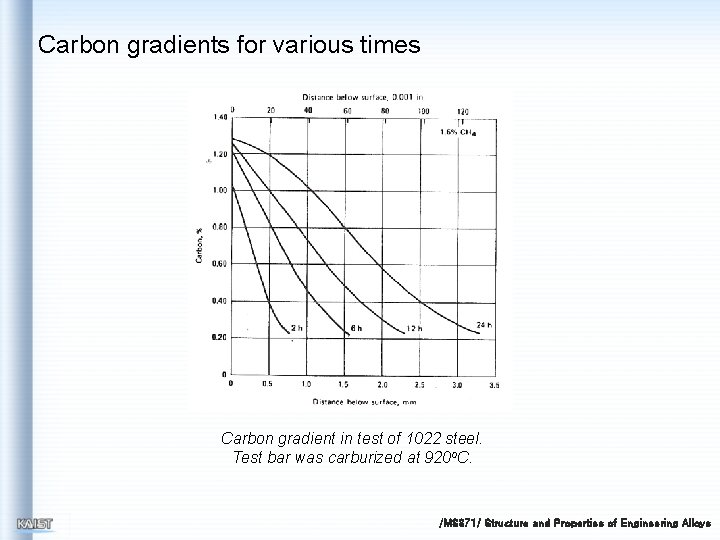
Carbon gradients for various times Carbon gradient in test of 1022 steel. Test bar was carburized at 920 o. C. /MS 371/ Structure and Properties of Engineering Alloys

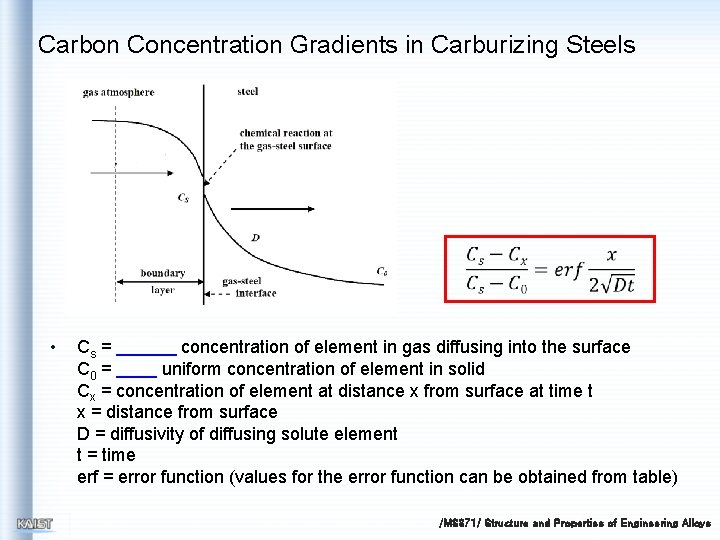
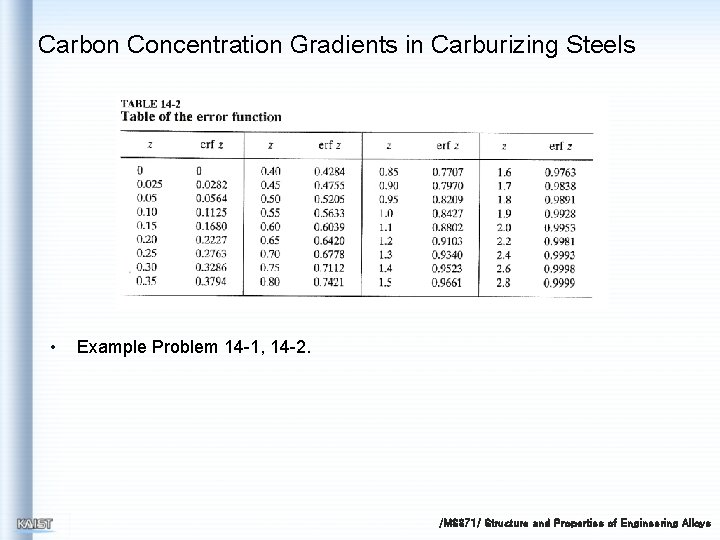
Carbon Concentration Gradients in Carburizing Steels • Cs = concentration of element in gas diffusing into the surface C 0 = uniform concentration of element in solid Cx = concentration of element at distance x from surface at time t x = distance from surface D = diffusivity of diffusing solute element t = time erf = error function (values for the error function can be obtained from table) /MS 371/ Structure and Properties of Engineering Alloys

Carbon Concentration Gradients in Carburizing Steels • Example Problem 14 -1, 14 -2. /MS 371/ Structure and Properties of Engineering Alloys

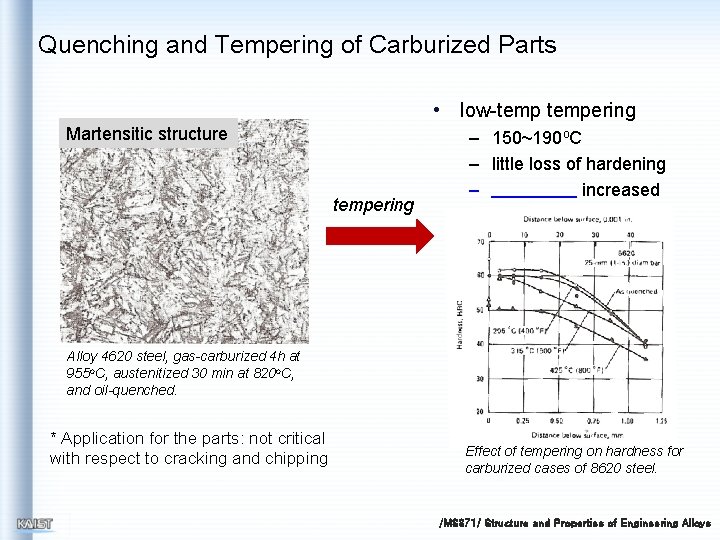
Quenching and Tempering of Carburized Parts • low-tempering Martensitic structure tempering – 150~190 o. C – little loss of hardening – increased Alloy 4620 steel, gas-carburized 4 h at 955 o. C, austenitized 30 min at 820 o. C, and oil-quenched. * Application for the parts: not critical with respect to cracking and chipping Effect of tempering on hardness for carburized cases of 8620 steel. /MS 371/ Structure and Properties of Engineering Alloys

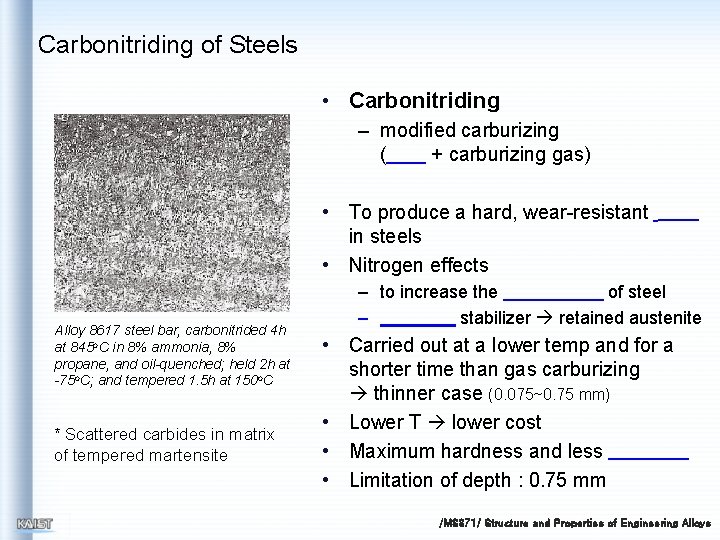
Carbonitriding of Steels • Carbonitriding – modified carburizing ( + carburizing gas) • To produce a hard, wear-resistant in steels • Nitrogen effects Alloy 8617 steel bar, carbonitrided 4 h at 845 o. C in 8% ammonia, 8% propane, and oil-quenched; held 2 h at -75 o. C; and tempered 1. 5 h at 150 o. C * Scattered carbides in matrix of tempered martensite – to increase the of steel – stabilizer retained austenite • Carried out at a lower temp and for a shorter time than gas carburizing thinner case (0. 075~0. 75 mm) • Lower T lower cost • Maximum hardness and less • Limitation of depth : 0. 75 mm /MS 371/ Structure and Properties of Engineering Alloys

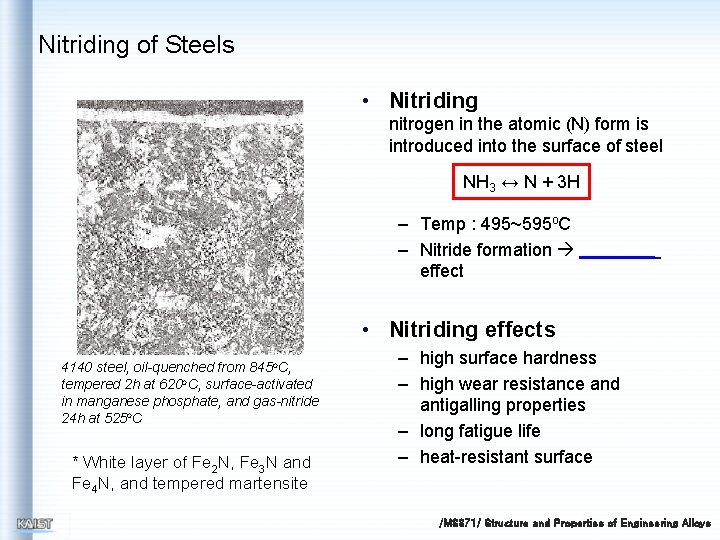
Nitriding of Steels • Nitriding nitrogen in the atomic (N) form is introduced into the surface of steel NH 3 ↔ N + 3 H – Temp : 495~595 o. C – Nitride formation effect • Nitriding effects 4140 steel, oil-quenched from 845 o. C, tempered 2 h at 620 o. C, surface-activated in manganese phosphate, and gas-nitride 24 h at 525 o. C * White layer of Fe 2 N, Fe 3 N and Fe 4 N, and tempered martensite – high surface hardness – high wear resistance and antigalling properties – long fatigue life – heat-resistant surface /MS 371/ Structure and Properties of Engineering Alloys

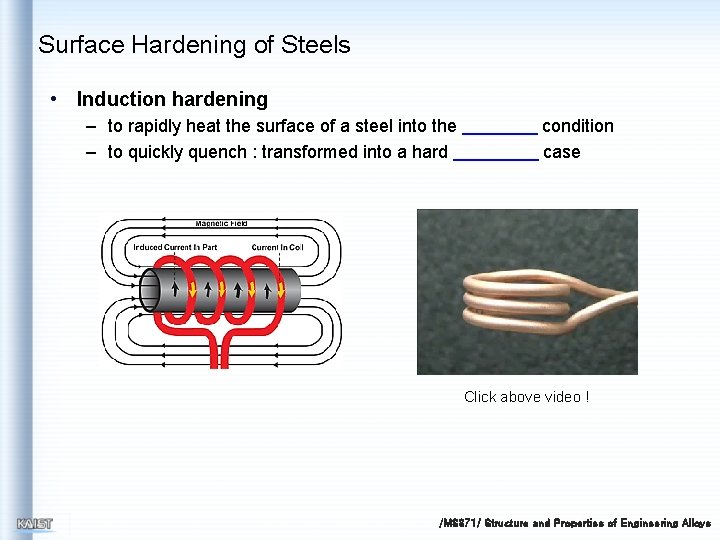
Surface Hardening of Steels • Induction hardening – to rapidly heat the surface of a steel into the – to quickly quench : transformed into a hard condition case Click above video ! /MS 371/ Structure and Properties of Engineering Alloys

Surface Hardening of Steels • Flame hardening – rapid and quick – for so large parts : large gears, dies, and rolls (not practical in a – for small sections : end of valve stems and push rods ) • Laser hardening – – intense heating workpiece itself to act as a cooling sink to harden a relatively small area in complex shapes but, high Flame hardening Laser hardening /MS 371/ Structure and Properties of Engineering Alloys

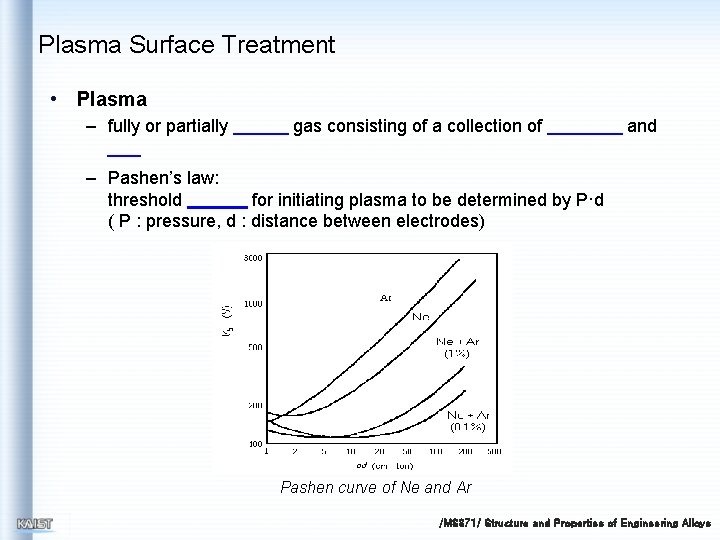
Plasma Surface Treatment • Plasma – fully or partially gas consisting of a collection of and – Pashen’s law: threshold for initiating plasma to be determined by P·d ( P : pressure, d : distance between electrodes) Pashen curve of Ne and Ar /MS 371/ Structure and Properties of Engineering Alloys

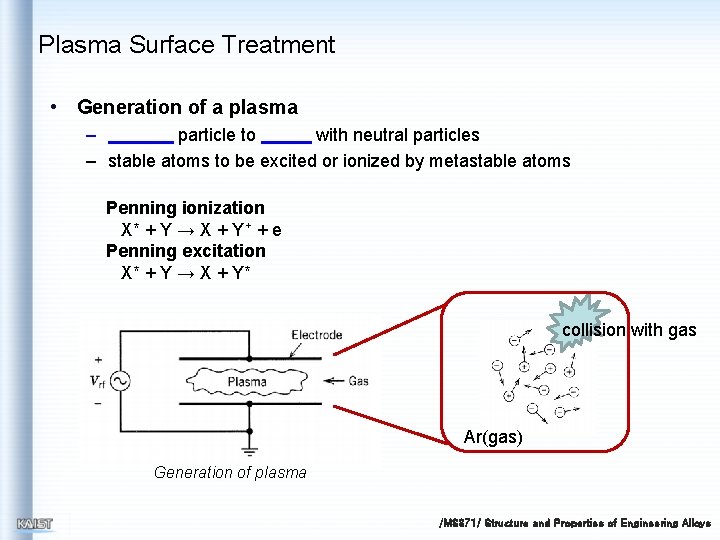
Plasma Surface Treatment • Generation of a plasma – particle to with neutral particles – stable atoms to be excited or ionized by metastable atoms Penning ionization X* + Y → X + Y+ + e Penning excitation X* + Y → X + Y* collision with gas Ar(gas) Generation of plasma /MS 371/ Structure and Properties of Engineering Alloys

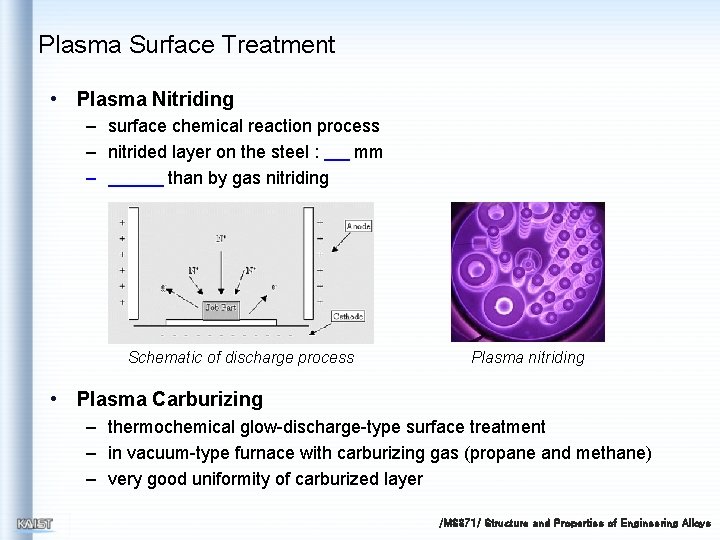
Plasma Surface Treatment • Plasma Nitriding – surface chemical reaction process – nitrided layer on the steel : mm – than by gas nitriding Schematic of discharge process Plasma nitriding • Plasma Carburizing – thermochemical glow-discharge-type surface treatment – in vacuum-type furnace with carburizing gas (propane and methane) – very good uniformity of carburized layer /MS 371/ Structure and Properties of Engineering Alloys

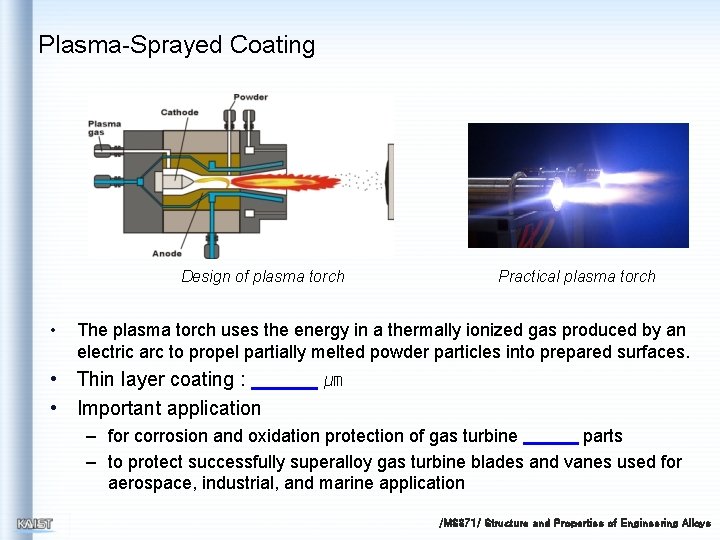
Plasma-Sprayed Coating Design of plasma torch • Practical plasma torch The plasma torch uses the energy in a thermally ionized gas produced by an electric arc to propel partially melted powder particles into prepared surfaces. • Thin layer coating : • Important application ㎛ – for corrosion and oxidation protection of gas turbine parts – to protect successfully superalloy gas turbine blades and vanes used for aerospace, industrial, and marine application /MS 371/ Structure and Properties of Engineering Alloys

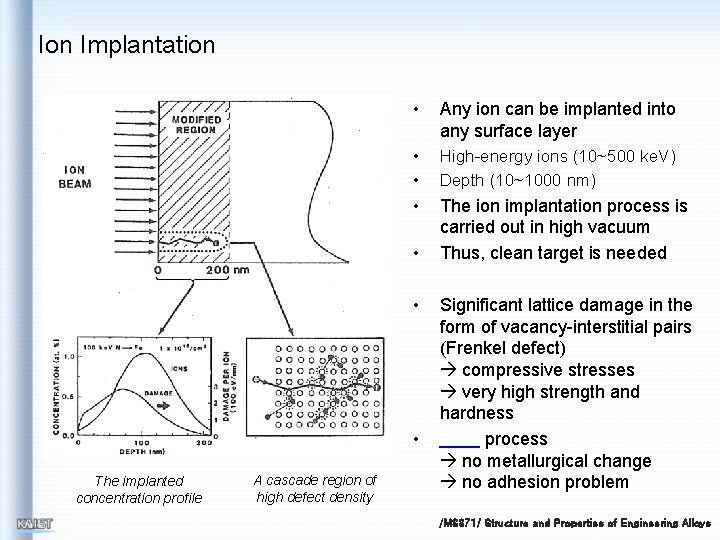
Ion Implantation • Any ion can be implanted into any surface layer • • High-energy ions (10~500 ke. V) Depth (10~1000 nm) • The ion implantation process is carried out in high vacuum Thus, clean target is needed • • • The implanted concentration profile A cascade region of high defect density Significant lattice damage in the form of vacancy-interstitial pairs (Frenkel defect) compressive stresses very high strength and hardness process no metallurgical change no adhesion problem /MS 371/ Structure and Properties of Engineering Alloys

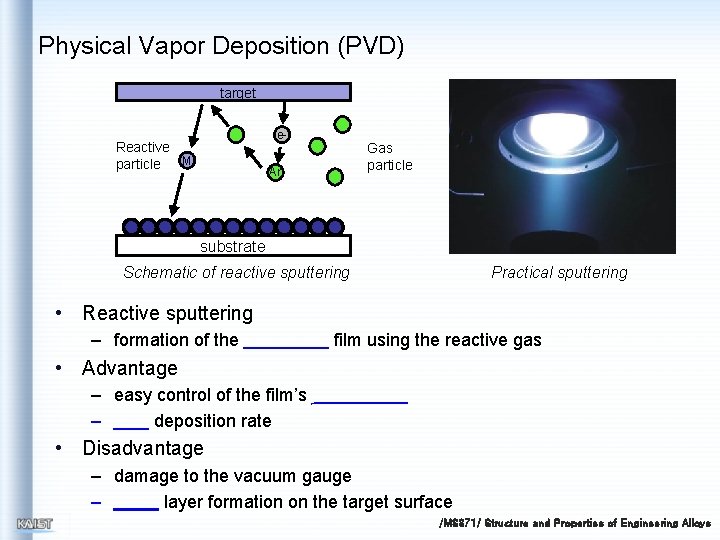
Physical Vapor Deposition (PVD) target e- Reactive M particle Gas particle Ar substrate Schematic of reactive sputtering Practical sputtering • Reactive sputtering – formation of the film using the reactive gas • Advantage – easy control of the film’s – deposition rate • Disadvantage – damage to the vacuum gauge – layer formation on the target surface /MS 371/ Structure and Properties of Engineering Alloys

Summary • Surface-hardening technique – gas carburizing, carbonitriding, and induction surface heating – for hard-wearing surface layer and tough inner cores • Localized surface-hardening technique – flame and laser hardening • Plasma carburizing & nitriding surface treatments • Plasma spray coating – for oxidation protection on Ni-base superalloys for gas turbines • Ion implantation and physical vapor deposition technique – for improved hardness and wear /MS 371/ Structure and Properties of Engineering Alloys