Ch 13 Solutions Give three examples of solutions
















- Slides: 23

Ch 13 Solutions • Give three examples of solutions in everyday life • What main components do these solution consist of? • How do you know that each of these examples is actually a solution?

Mixtures • Solutions- Homogeneous mixture of two or more substances uniformly dispersed throughout a single phase • Suspensions- mixture in which particles are temporarily heterogeneous mixed. They will settle and separate • Colloid- heterogeneous mixture that is stable and does not settle

Solutions • Two parts– Solvent – the substance in which the solute is dissolved – Solute- the substance that is dissolved in the solvent Ex. Salt water: Solvent – water solute - salt

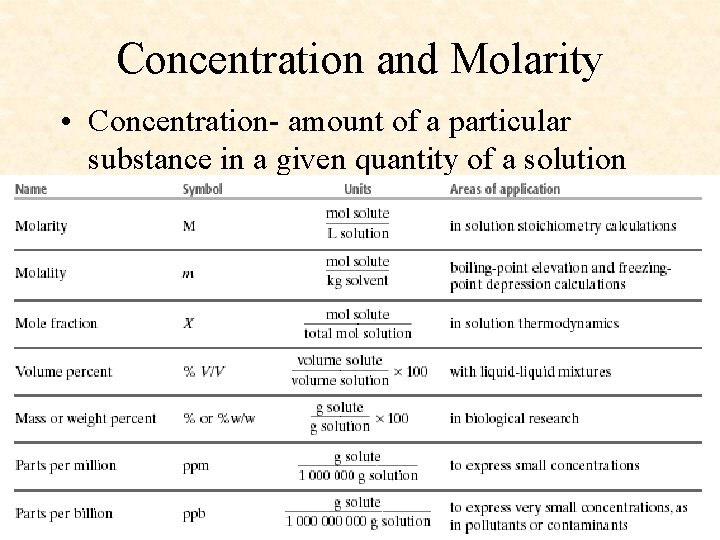
Concentration and Molarity • Concentration- amount of a particular substance in a given quantity of a solution

Concentration • Amount of a solute dissolved in a given quantity of solvent • Expressed as – Molarity – Molality – Parts per million – Parts per billion

Calculating parts per million • Grams of solute in 1000000 grams of solvent • If there are 2. 2 mg of Hg in 500 g of a water sample, what is the concentration in ppm? • 2. 2 mg = ? g • 0. 0022 g Hg x 1000000 parts 500 g H 2 O 1 million 4. 44 ppm

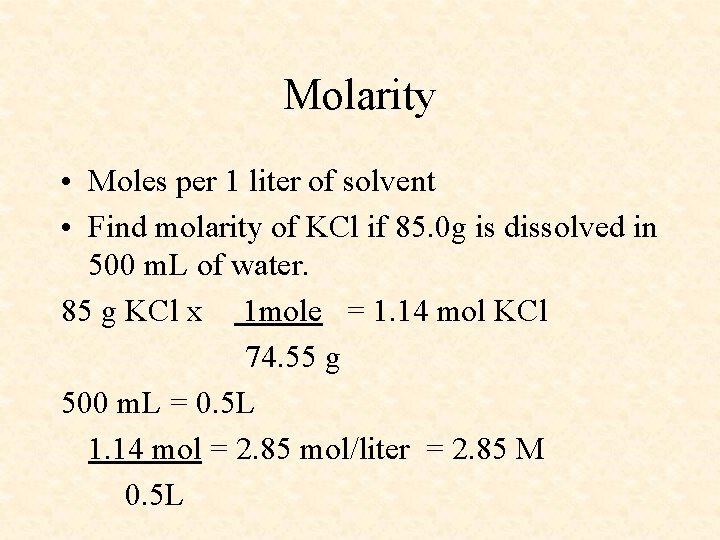
Molarity • Moles per 1 liter of solvent • Find molarity of KCl if 85. 0 g is dissolved in 500 m. L of water. 85 g KCl x 1 mole = 1. 14 mol KCl 74. 55 g 500 m. L = 0. 5 L 1. 14 mol = 2. 85 mol/liter = 2. 85 M 0. 5 L

Solvationprocess of creating a solution ionic compound(solute) dissociates to cation and anion and dissolve in water (polar) Like dissolves likepolar solvent dissolve charged solutes non-polar solvents dissolve nonpolar molecules

Separating mixtures • • • Filtering – heterogeneous mixtures Centrifuging- heterogeneous mixtures Decanting- heterogeneous mixtures Evaporation- homogenous mixtures Distillation- homogeneous mixtures Chromatography- homogeneous mixtures

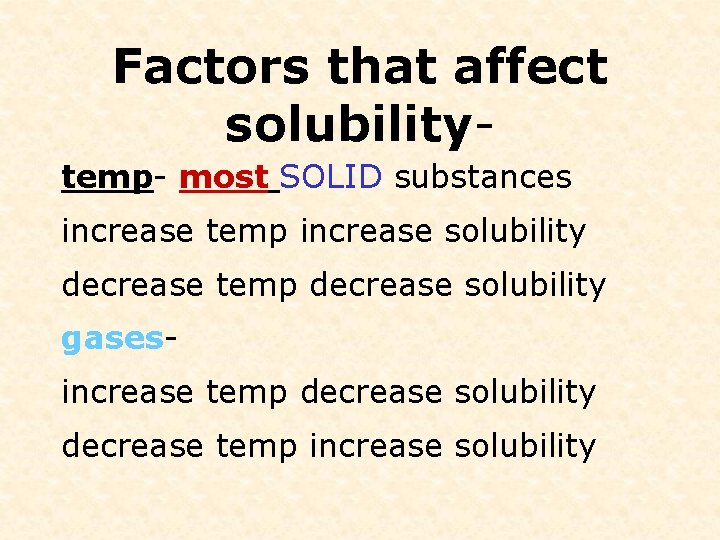
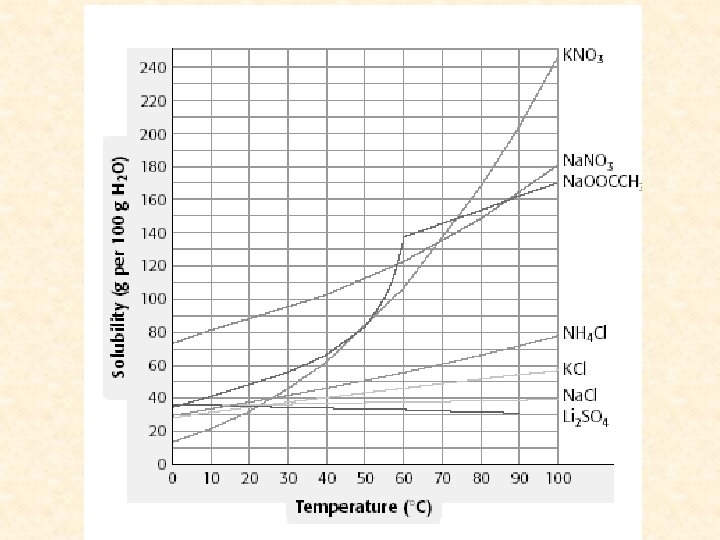
Factors that affect solubilitytemp- most SOLID substances increase temp increase solubility decrease temp decrease solubility gasesincrease temp decrease solubility decrease temp increase solubility


Pressuregases- increase pressure increase solubility solids- no affect liquids- very slight affect

Surface Area • Greater surface area speeds up dissolving process • Crush solids to increase surface area

Saturation A. Saturated- most possible solute at temp B. Unsaturated- less than maximum at temp C. Supersaturated- more than max possible at temp (heat then cool)

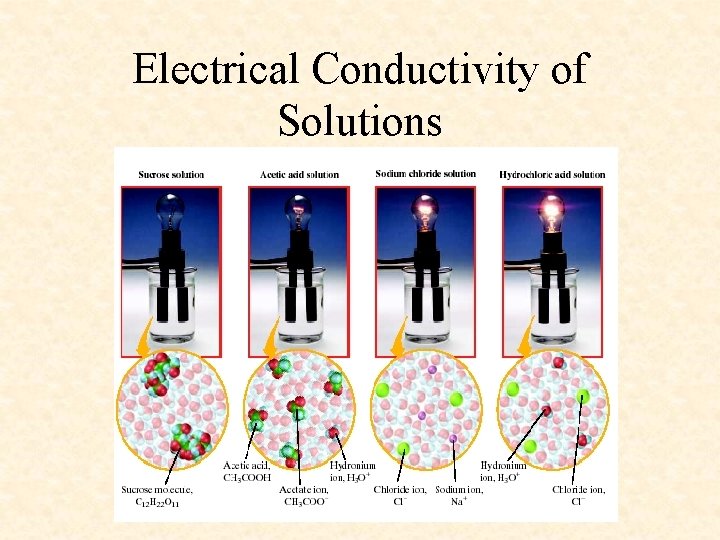
Physical Properties of Solutions • Electrolytes- substance that dissolves in a liquid and provides ions that conduct electricity • Ex. - Gatorade, etc

Electrical Conductivity of Solutions

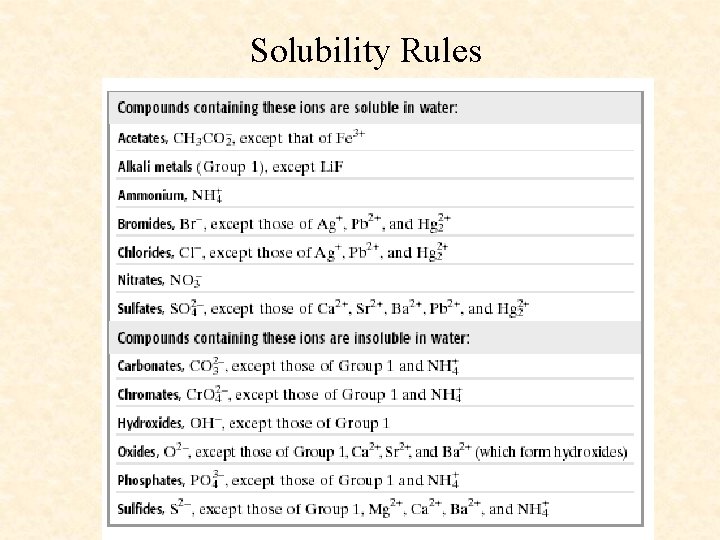
Solubility Rules

Colligitive properties of solutions Boiling point elevationthe bp of solutions is different from pure substances increase concentration of solution the greater the bp is elevated different solutes change the bp by different amounts

Freezing point depressionthe fp of solutions is different than the fp of pure substances increase concentration of solution the lower the fp of the solution decrease the concentration of the solution and the less the fp is depressed

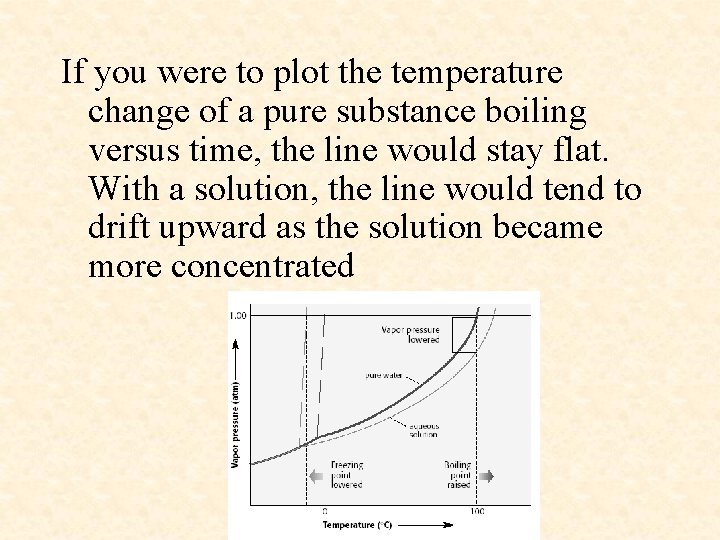
• Pure substances have true boiling points and freezing points, but solutions do not. For example, pure water has a boiling point of 100 °C and a freezing point of 0 °C. In boiling for example, as pure water vapor leaves the liquid, only pure water is left behind. Not so with a solution.

If you were to plot the temperature change of a pure substance boiling versus time, the line would stay flat. With a solution, the line would tend to drift upward as the solution became more concentrated

• As a solution boils, if the solute is non-volatile, then only pure solvent enters the vapor phase. The solute stays behind (this is the meaning of non-volatile). However, the consequence is that the solution becomes more concentrated, hence its boiling point increases.

Molalitynumber of moles of solute per kilogram of solvent used to calculate bp and fp changes