Ch 13 Gases Liquids Solids Phase Changes Kinetic





- Slides: 22

Ch. 13 – Gases, Liquids Solids & Phase Changes

Kinetic Molecular Theory u Particles • • • in an ideal gas… have no volume. have elastic collisions. are in constant, random, straight-line motion. don’t attract or repel each other. have an avg. KE directly related to Kelvin temperature.

Real Gases u Particles in a REAL gas… • have their own volume • attract each other u Gas behavior is most ideal… • at low pressures • at high temperatures • in nonpolar atoms/molecules

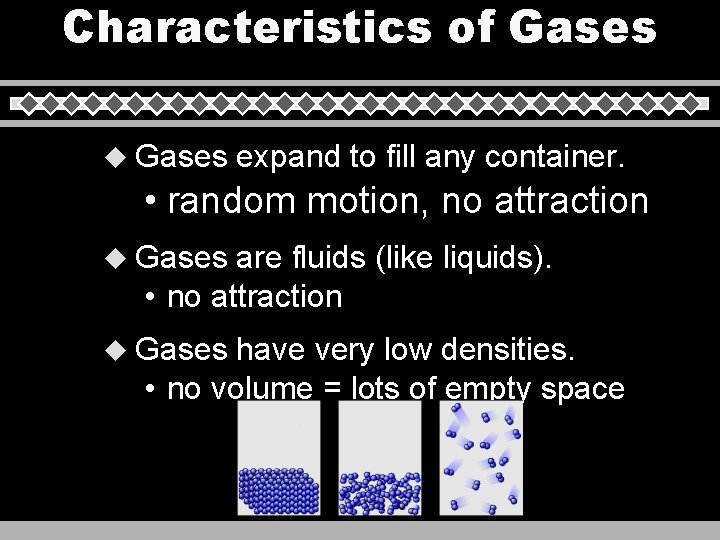
Characteristics of Gases u Gases expand to fill any container. • random motion, no attraction u Gases are fluids (like liquids). • no attraction u Gases have very low densities. • no volume = lots of empty space

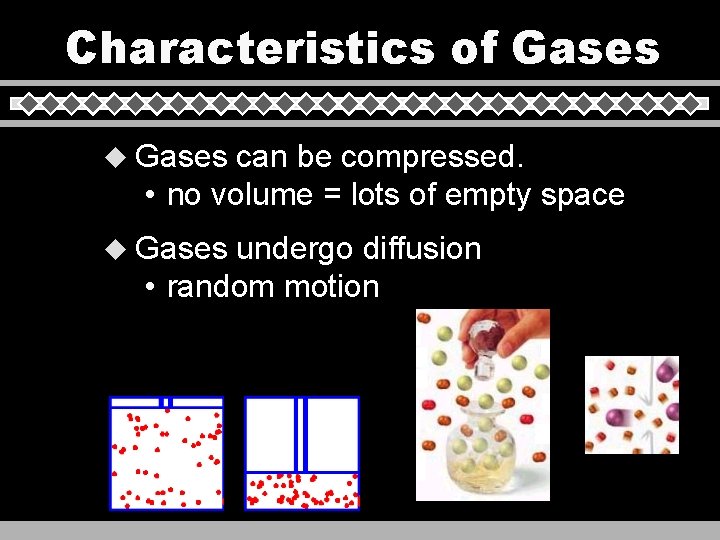
Characteristics of Gases u Gases can be compressed. • no volume = lots of empty space u Gases undergo diffusion • random motion

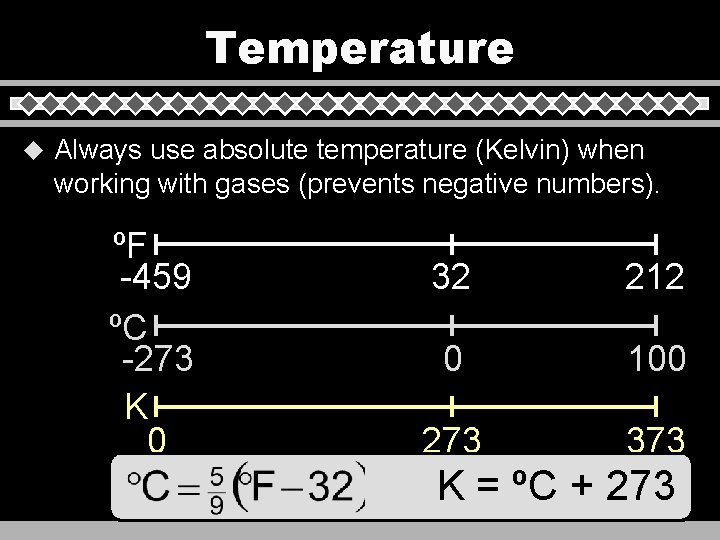
Temperature u Always use absolute temperature (Kelvin) when working with gases (prevents negative numbers). ºF -459 ºC -273 K 0 32 212 0 100 273 373 K = ºC + 273

Pressure Which shoes create the most pressure?

Pressure u Barometer • measures atmospheric pressure Mercury Barometer

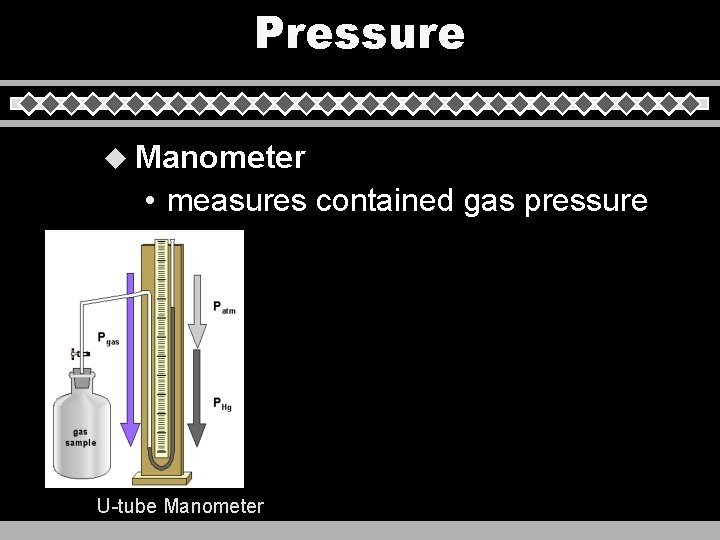
Pressure u Manometer • measures contained gas pressure U-tube Manometer

Pressure u KEY UNITS AT SEA LEVEL 101. 325 k. Pa (kilopascal) 1 atm 760 mm Hg 760 torr 14. 7 psi

STP Standard Temperature & Pressure 0°C 1 atm -OR- 273 K 101. 325 k. Pa

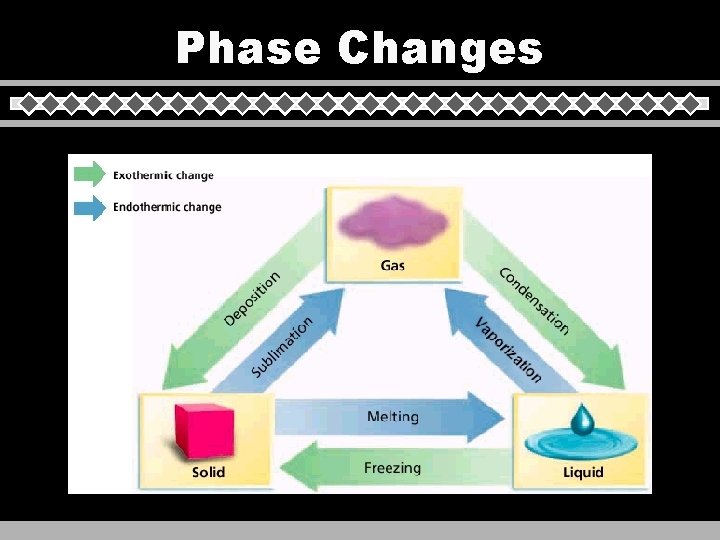
Phase Changes

Phase Changes u Evaporation • molecules at the surface gain enough energy to overcome IMF u Volatility • measure of evaporation rate • depends on temp & IMF

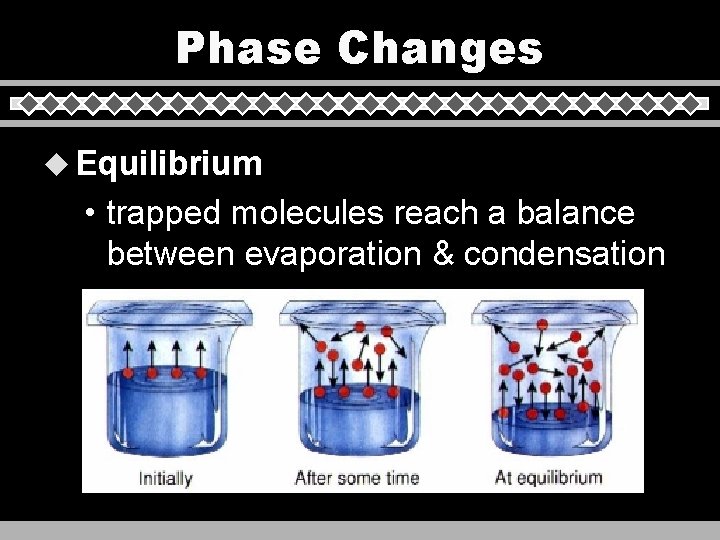
Phase Changes u Equilibrium • trapped molecules reach a balance between evaporation & condensation

Heating Curves u Temperature Change • change in KE (molecular motion) • depends on heat capacity u Heat Capacity • energy required to raise the temp of 1 gram of a substance by 1°C

Heating Curves u Phase Change • change in PE (molecular arrangement) • temp remains constant u All six phase changes can occur at the triple point: freezing and melting, evaporation and condensation, sublimation and deposition. C. Johannesson

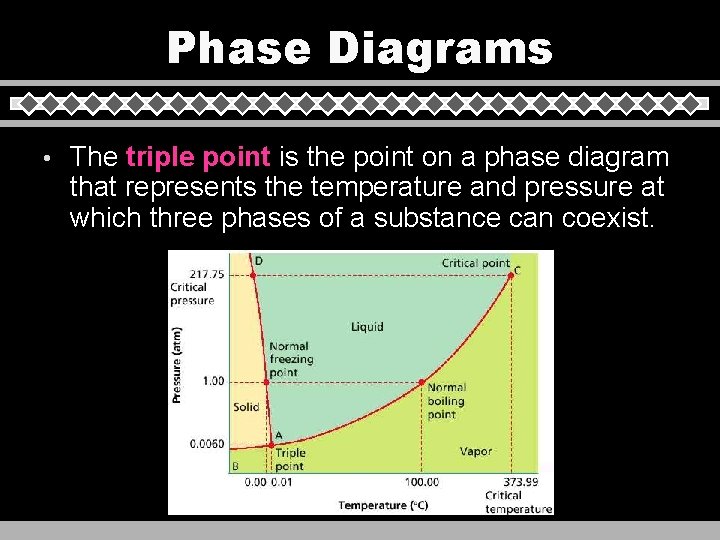
Phase Diagrams • The triple point is the point on a phase diagram that represents the temperature and pressure at which three phases of a substance can coexist.

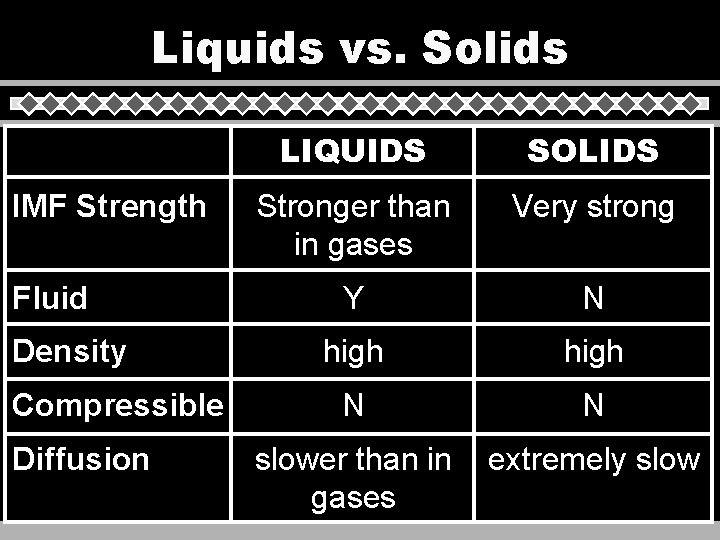
Liquids vs. Solids IMF Strength Fluid Density Compressible Diffusion LIQUIDS SOLIDS Stronger than in gases Very strong Y N high N N slower than in gases extremely slow

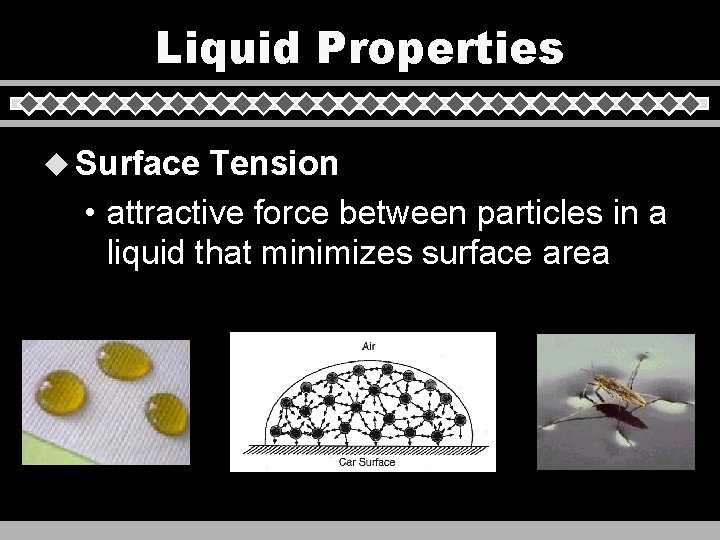
Liquid Properties u Surface Tension • attractive force between particles in a liquid that minimizes surface area

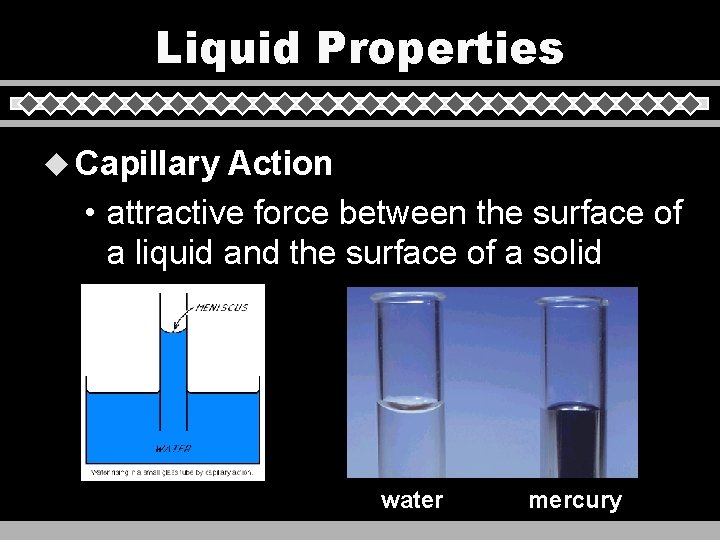
Liquid Properties u Capillary Action • attractive force between the surface of a liquid and the surface of a solid water mercury

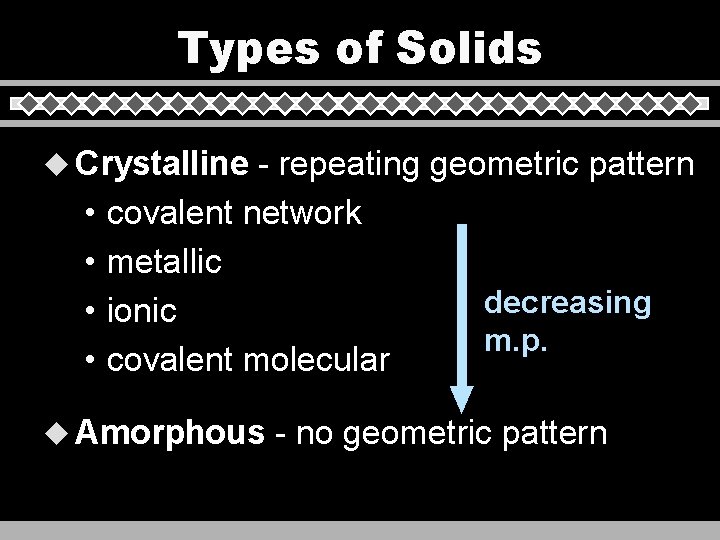
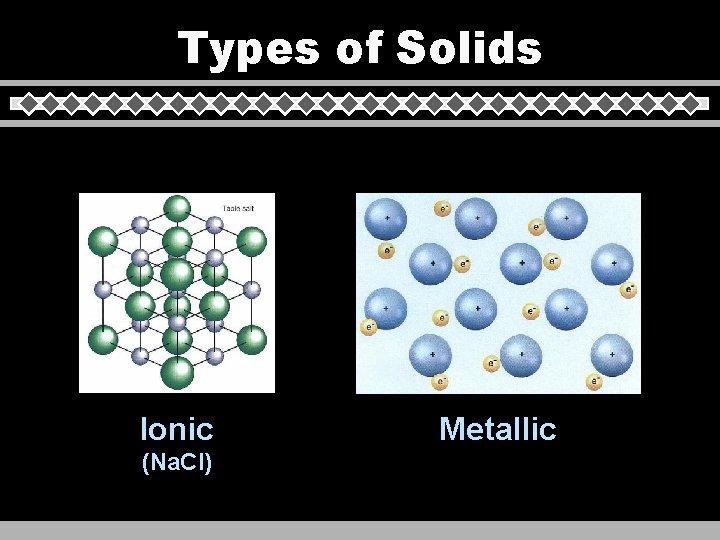
Types of Solids u Crystalline • • - repeating geometric pattern covalent network metallic decreasing ionic m. p. covalent molecular u Amorphous - no geometric pattern

Types of Solids Ionic (Na. Cl) Metallic