Ch 12 Inorganic Reaction Mechanisms I Introduction A
![3) 4) E. If [Y] is large, both become first order expressions For both 3) 4) E. If [Y] is large, both become first order expressions For both](https://slidetodoc.com/presentation_image_h2/a3b12d56689b63c4541de36b2532efb3/image-8.jpg)
- Slides: 13

Ch 12 Inorganic Reaction Mechanisms I. Introduction A. Parallels to Organic Chemistry 1) Many terms and concepts are the same as in organic mechanisms 2) Complex geometries are more common in inorganic complexes a) More rearrangements are possible b) More isomers are possible 3) Not all metal ions react alike; all carbon atoms do B. C. History and Goals 1) Werner and Jorgenson discovered many of the basic reactions 2) Experimentation over many years has yielded proposed mechanisms 3) Mechanisms can’t be proven, only disproven a) We can’t directly observe individual molecules react b) Evidence either supports a mechanism or rules it out 4) Goal: synthesize the predicted products by choosing the appropriate reaction conditions. Types of Reactions: Substitution, Oxidation/Reduction, Ligand Reactions

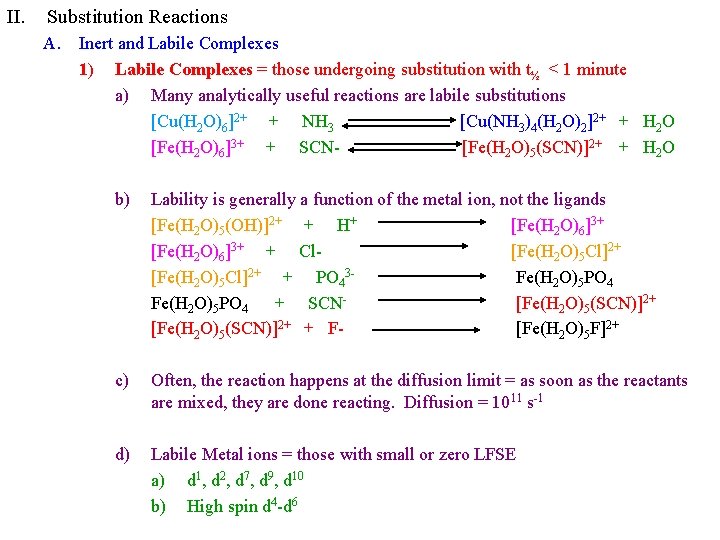
II. Substitution Reactions A. Inert and Labile Complexes 1) Labile Complexes = those undergoing substitution with t½ < 1 minute a) Many analytically useful reactions are labile substitutions [Cu(H 2 O)6]2+ + NH 3 [Cu(NH 3)4(H 2 O)2]2+ + H 2 O [Fe(H 2 O)6]3+ + SCN[Fe(H 2 O)5(SCN)]2+ + H 2 O b) Lability is generally a function of the metal ion, not the ligands [Fe(H 2 O)5(OH)]2+ + H+ [Fe(H 2 O)6]3+ + Cl[Fe(H 2 O)5 Cl]2+ + PO 43 Fe(H 2 O)5 PO 4 + SCN[Fe(H 2 O)5(SCN)]2+ + F[Fe(H 2 O)5 F]2+ c) Often, the reaction happens at the diffusion limit = as soon as the reactants are mixed, they are done reacting. Diffusion = 1011 s-1 d) Labile Metal ions = those with small or zero LFSE a) d 1, d 2, d 7, d 9, d 10 b) High spin d 4 -d 6

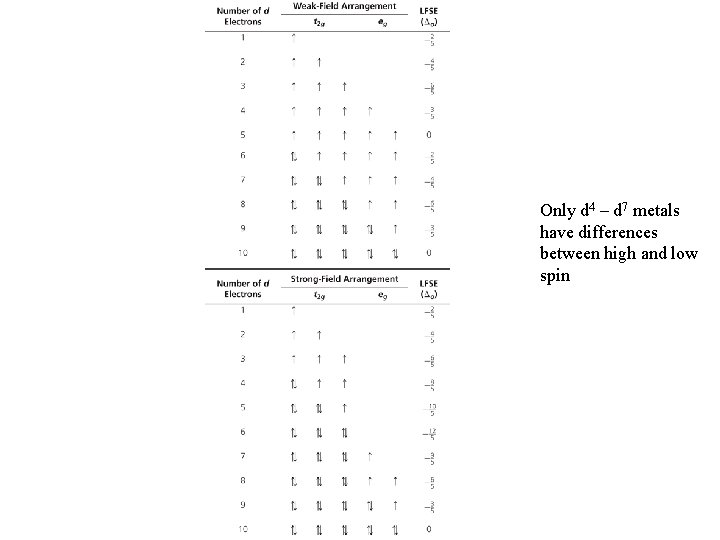
Only d 4 – d 7 metals have differences between high and low spin

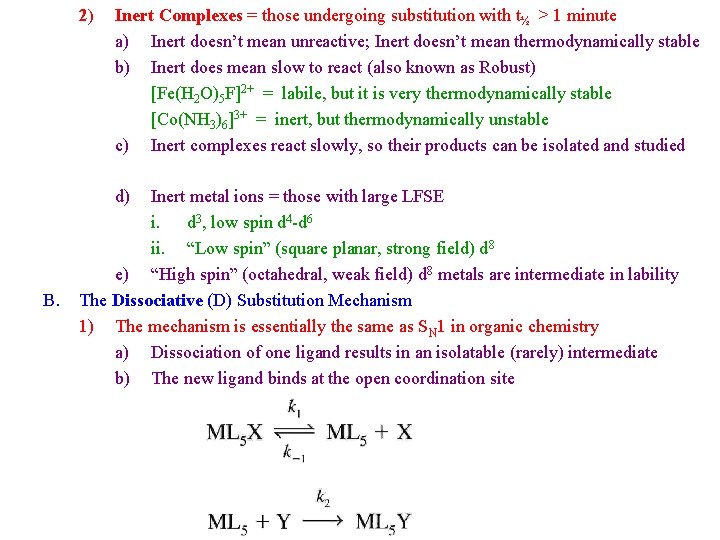
2) Inert Complexes = those undergoing substitution with t½ > 1 minute a) Inert doesn’t mean unreactive; Inert doesn’t mean thermodynamically stable b) Inert does mean slow to react (also known as Robust) [Fe(H 2 O)5 F]2+ = labile, but it is very thermodynamically stable [Co(NH 3)6]3+ = inert, but thermodynamically unstable c) Inert complexes react slowly, so their products can be isolated and studied d) B. Inert metal ions = those with large LFSE i. d 3, low spin d 4 -d 6 ii. “Low spin” (square planar, strong field) d 8 e) “High spin” (octahedral, weak field) d 8 metals are intermediate in lability The Dissociative (D) Substitution Mechanism 1) The mechanism is essentially the same as SN 1 in organic chemistry a) Dissociation of one ligand results in an isolatable (rarely) intermediate b) The new ligand binds at the open coordination site

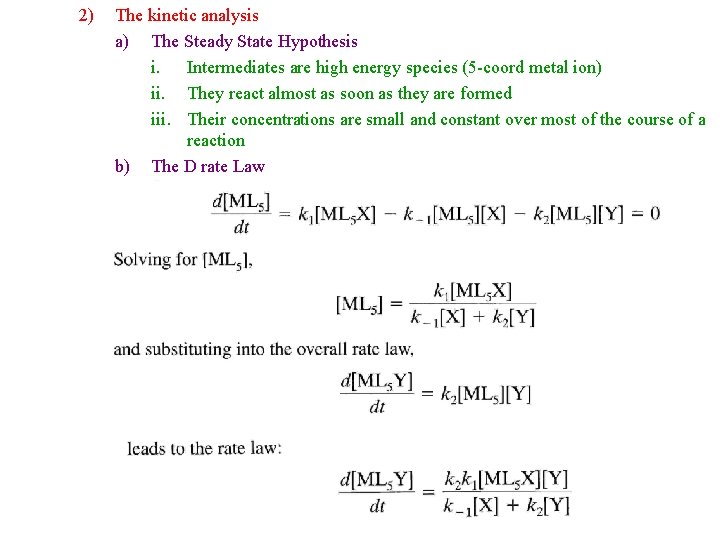
2) The kinetic analysis a) The Steady State Hypothesis i. Intermediates are high energy species (5 -coord metal ion) ii. They react almost as soon as they are formed iii. Their concentrations are small and constant over most of the course of a reaction b) The D rate Law

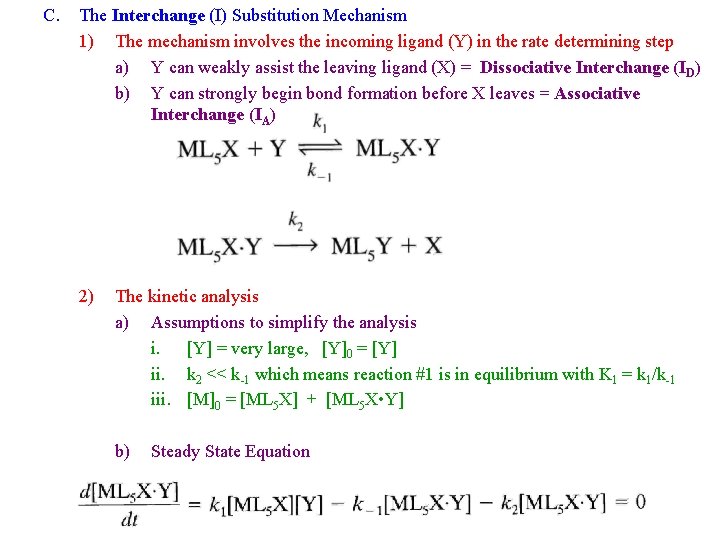
C. The Interchange (I) Substitution Mechanism 1) The mechanism involves the incoming ligand (Y) in the rate determining step a) Y can weakly assist the leaving ligand (X) = Dissociative Interchange (ID) b) Y can strongly begin bond formation before X leaves = Associative Interchange (IA) 2) The kinetic analysis a) Assumptions to simplify the analysis i. [Y] = very large, [Y]0 = [Y] ii. k 2 << k-1 which means reaction #1 is in equilibrium with K 1 = k 1/k-1 iii. [M]0 = [ML 5 X] + [ML 5 X • Y] b) Steady State Equation

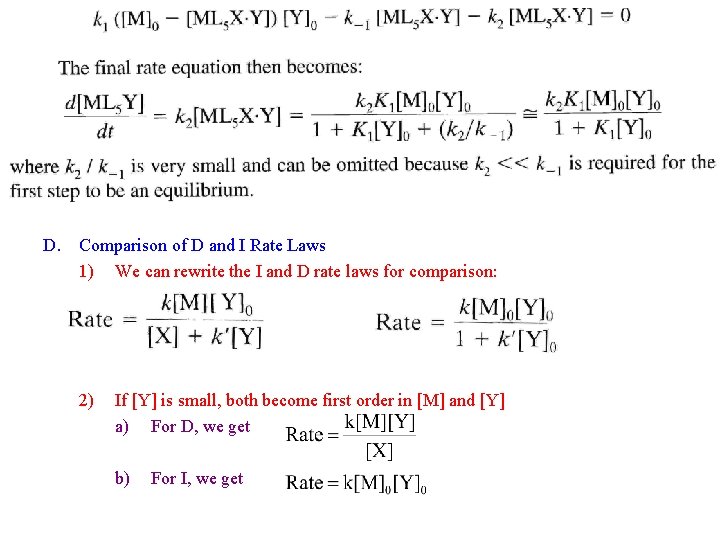
D. Comparison of D and I Rate Laws 1) We can rewrite the I and D rate laws for comparison: 2) If [Y] is small, both become first order in [M] and [Y] a) For D, we get b) For I, we get
![3 4 E If Y is large both become first order expressions For both 3) 4) E. If [Y] is large, both become first order expressions For both](https://slidetodoc.com/presentation_image_h2/a3b12d56689b63c4541de36b2532efb3/image-8.jpg)
3) 4) E. If [Y] is large, both become first order expressions For both I and D, we get These similarities make it difficult to ever tell these two mechanisms apart based on experimental data a) Usually, we vary the concentration of [M], [Y], or [X] to find the order b) Isolation of ML 5 is proof of the D mechanism The Associative (A) Substitution Mechanism 1) The mechanism is essentially the SN 2 mechanism from Organic chemistry a) Y and X are both partially bonded to M at the transition state b) An Intermediate is only rarely isolated 2) Most reaction best fit between D and A, into the ID and IA mechanisms

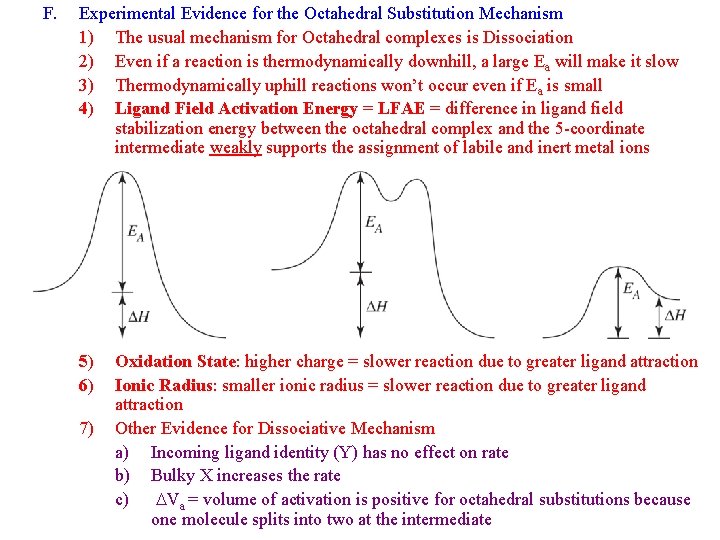
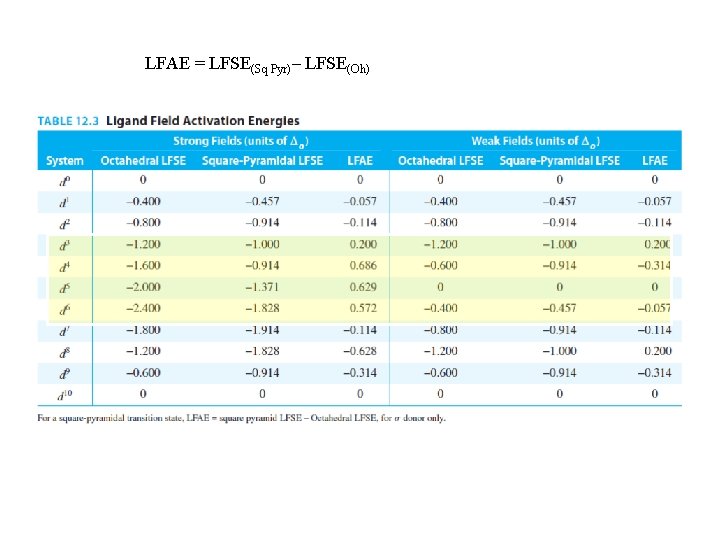
F. Experimental Evidence for the Octahedral Substitution Mechanism 1) The usual mechanism for Octahedral complexes is Dissociation 2) Even if a reaction is thermodynamically downhill, a large Ea will make it slow 3) Thermodynamically uphill reactions won’t occur even if Ea is small 4) Ligand Field Activation Energy = LFAE = difference in ligand field stabilization energy between the octahedral complex and the 5 -coordinate intermediate weakly supports the assignment of labile and inert metal ions 5) 6) 7) Oxidation State: higher charge = slower reaction due to greater ligand attraction Ionic Radius: smaller ionic radius = slower reaction due to greater ligand attraction Other Evidence for Dissociative Mechanism a) Incoming ligand identity (Y) has no effect on rate b) Bulky X increases the rate c) DVa = volume of activation is positive for octahedral substitutions because one molecule splits into two at the intermediate

LFAE = LFSE(Sq Pyr)– LFSE(Oh)

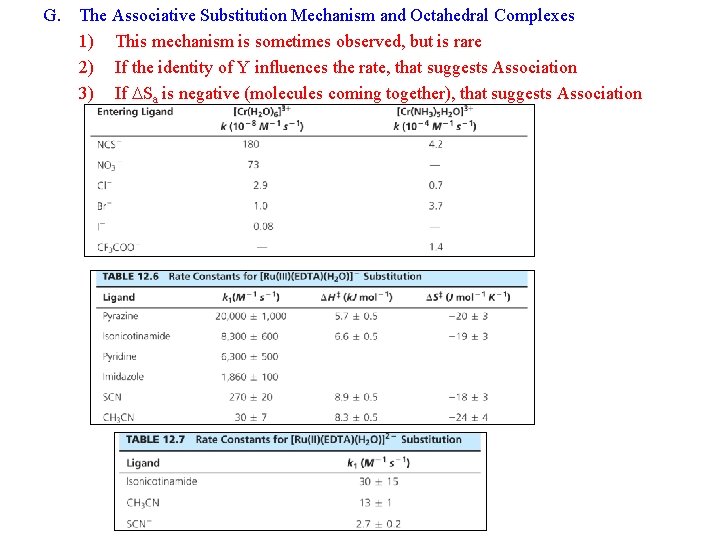
G. The Associative Substitution Mechanism and Octahedral Complexes 1) This mechanism is sometimes observed, but is rare 2) If the identity of Y influences the rate, that suggests Association 3) If DSa is negative (molecules coming together), that suggests Association

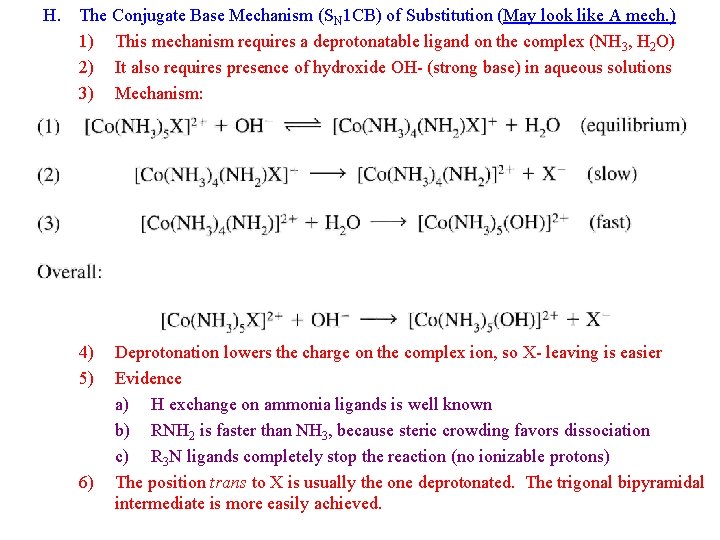
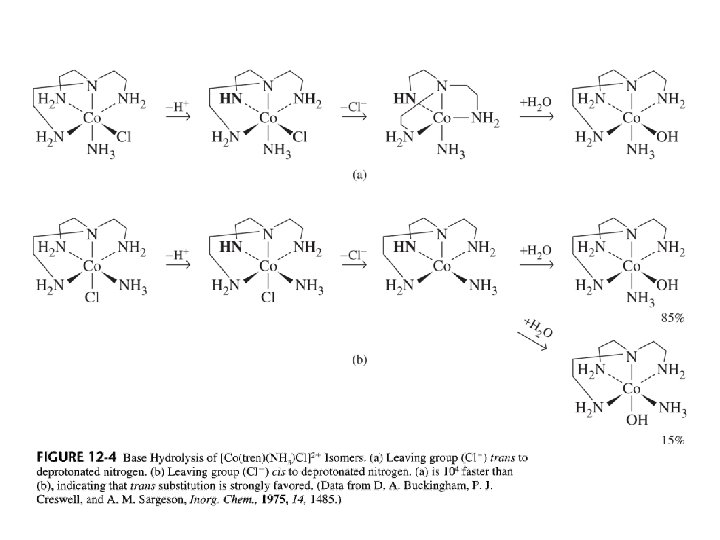
H. The Conjugate Base Mechanism (SN 1 CB) of Substitution (May look like A mech. ) 1) This mechanism requires a deprotonatable ligand on the complex (NH 3, H 2 O) 2) It also requires presence of hydroxide OH- (strong base) in aqueous solutions 3) Mechanism: 4) 5) 6) Deprotonation lowers the charge on the complex ion, so X- leaving is easier Evidence a) H exchange on ammonia ligands is well known b) RNH 2 is faster than NH 3, because steric crowding favors dissociation c) R 3 N ligands completely stop the reaction (no ionizable protons) The position trans to X is usually the one deprotonated. The trigonal bipyramidal intermediate is more easily achieved.

 Organic chemistry
Organic chemistry Inorganic pharmaceutical
Inorganic pharmaceutical Introduction to inorganic chemistry
Introduction to inorganic chemistry Reaction rate equation
Reaction rate equation Addition reaction and substitution reaction
Addition reaction and substitution reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Proton capture equation
Proton capture equation Smear layer definition
Smear layer definition Charring test of organic and inorganic compounds
Charring test of organic and inorganic compounds Inorganic plant
Inorganic plant Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Neon organic or inorganic
Neon organic or inorganic Which compound is inorganic
Which compound is inorganic Importance of organic compounds
Importance of organic compounds
























