CH 104 DETERMINATION OF AN EQUILIBRIUM CONSTANT EQUILIBRIUM

CH 104: DETERMINATION OF AN EQUILIBRIUM CONSTANT EQUILIBRIUM • Hydrogen (H 2) and iodine (I 2) react to produce hydroiodic acid (HI). Forward Reaction: H 2(g) + I 2(g) → 2 HI(g) • However, as soon as some HI is produced it begins to dissociate back to H 2 and I 2. Reverse Reaction: 2 HI(g) → H 2(g) + I 2(g) • These 2 opposing reactions, 1 forward and 1 reverse, go on simultaneously. Ultimately, the rate of the forward reaction will equal the rate of the reverse reaction and the system is in dynamic equilibrium. H 2(g) + I 2(g) = 2 HI(g)

EQUILIBRIUM • • How many moles of H 2(g), I 2(g), and HI(g) are initially used in experiment 1? H 2(g) = 0. 0015 moles, I 2(g) = 0. 0015 moles, and HI(g) = 0 moles. How many moles of H 2(g), I 2(g), and HI(g) are initially used in experiment 2? H 2(g) = 0 moles, I 2(g) = 0 moles, and HI(g) = 0. 0015 moles. How many moles of H 2(g), I 2(g), and HI(g) are initially used in experiment 3? H 2(g) = 0. 0015 moles, I 2(g) = 0. 0015 moles, and HI(g) = 0. 0015 moles. In summary, all 3 experiments reach a dynamic equilibrium regardless of the initial concentrations of H 2(g), I 2(g), and HI(g).

EQUILIBRIUM H 2(g) + I 2(g) = 2 HI(g) • At equilibrium the rate of the forward reaction equals the rate of the reverse reaction. H 2(g) and I 2(g) are still producing HI(g). And HI(g) is still producing H 2(g) and I 2(g). However, the final concentrations of H 2(g), I 2(g), and HI(g) are constant.

EQUILIBRIUM • If the rate of people going to the first floor equals the rate of people going to the second floor, the number of people on each floor remains constant and the 2 groups are at dynamic equilibrium.
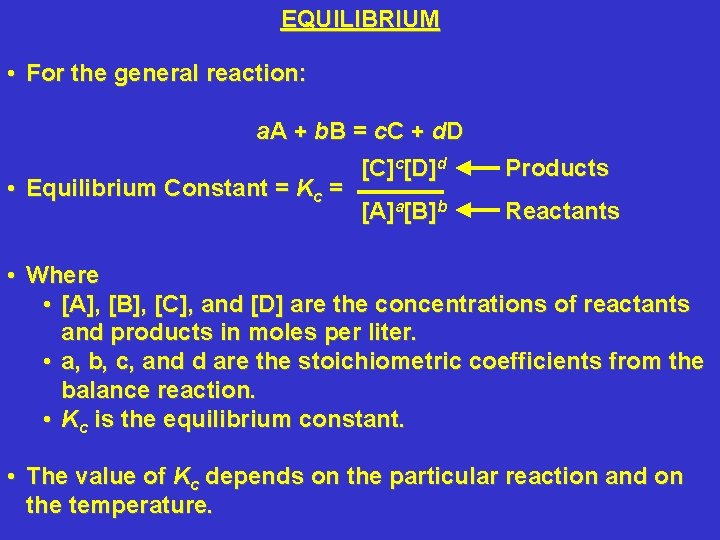
EQUILIBRIUM • For the general reaction: a. A + b B = c. C + d D • Equilibrium Constant = Kc = [C]c[D]d Products [A]a[B]b Reactants • Where • [A], [B], [C], and [D] are the concentrations of reactants and products in moles per liter. • a, b, c, and d are the stoichiometric coefficients from the balance reaction. • Kc is the equilibrium constant. • The value of Kc depends on the particular reaction and on the temperature.
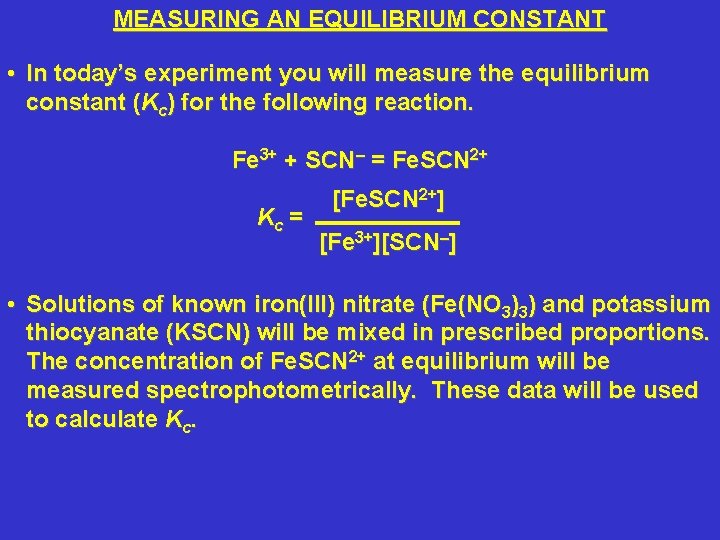
MEASURING AN EQUILIBRIUM CONSTANT • In today’s experiment you will measure the equilibrium constant (Kc) for the following reaction. Fe 3+ + SCN– = Fe. SCN 2+ Kc = [Fe. SCN 2+] [Fe 3+][SCN–] • Solutions of known iron(III) nitrate (Fe(NO 3)3) and potassium thiocyanate (KSCN) will be mixed in prescribed proportions. The concentration of Fe. SCN 2+ at equilibrium will be measured spectrophotometrically. These data will be used to calculate Kc.

MEASURING AN EQUILIBRIUM CONSTANT • If 10. 00 m. L of 0. 00200 M Fe(NO 3)3, 5. 00 m. L of 0. 00200 M KSCN, and 5. 00 m. L of distilled water are mixed together, what is the initial concentration of Fe 3+? • [Fe 3+]initial = 0. 00100 M • What is the initial concentration of SCN–? • [SCN–]initial = 0. 000500 M • What is the initial concentration of Fe. SCN 2+? • [Fe. SCN 2+]initial = 0 M • If the concentration of Fe. SCN 2+ at equilibrium is 0. 000120 M, what is the equilibrium concentration of Fe 3+? Fe 3+ + SCN– = Fe. SCN 2+ • [Fe 3+]equilibrium = 0. 00100 M – 0. 000120 M = 0. 00088 M • What is the equilibrium concentration of SCN–? • [SCN–]equilibrium = 0. 000500 M – 0. 000120 M = 0. 000380 M

MEASURING AN EQUILIBRIUM CONSTANT • An ICE (Initial, Change, Equilibrium) table is used to solve these types of problems. Reaction: Fe 3+ + Initial concentrations: 0. 00100 M 0. 000500 M 0 M – 0. 000120 M + 0. 000120 M 0. 00088 M 0. 000380 M 0. 000120 M Change: Equilibrium concentrations: SCN– = • What is the equilibrium constant? [Fe. SCN 2+] Kc = [Fe 3+][SCN–] Kc = [0. 000120] [0. 00088][0. 000380] = 3. 6 x 10 2 Fe. SCN 2+
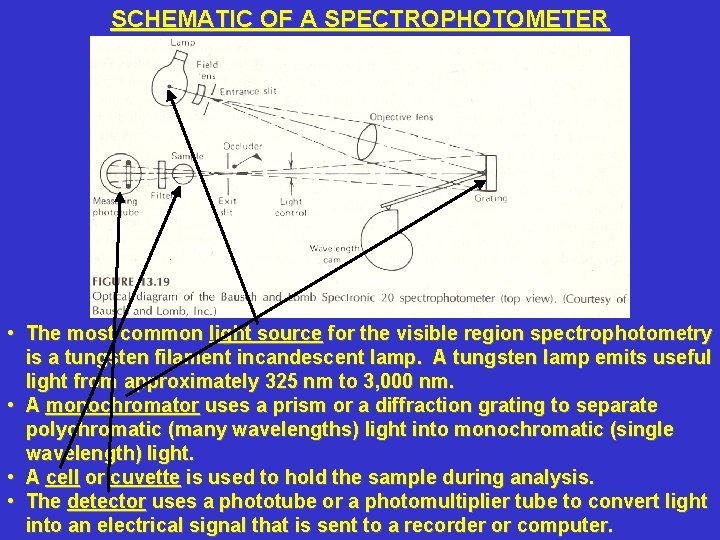
SCHEMATIC OF A SPECTROPHOTOMETER • The most common light source for the visible region spectrophotometry is a tungsten filament incandescent lamp. A tungsten lamp emits useful light from approximately 325 nm to 3, 000 nm. • A monochromator uses a prism or a diffraction grating to separate polychromatic (many wavelengths) light into monochromatic (single wavelength) light. • A cell or cuvette is used to hold the sample during analysis. • The detector uses a phototube or a photomultiplier tube to convert light into an electrical signal that is sent to a recorder or computer.

THE SPECTRONIC 20 D SPECTROPHOTOMETER The controls. Loading a sample. THE HACH DR 2010 SPECTROPHOTOMETER
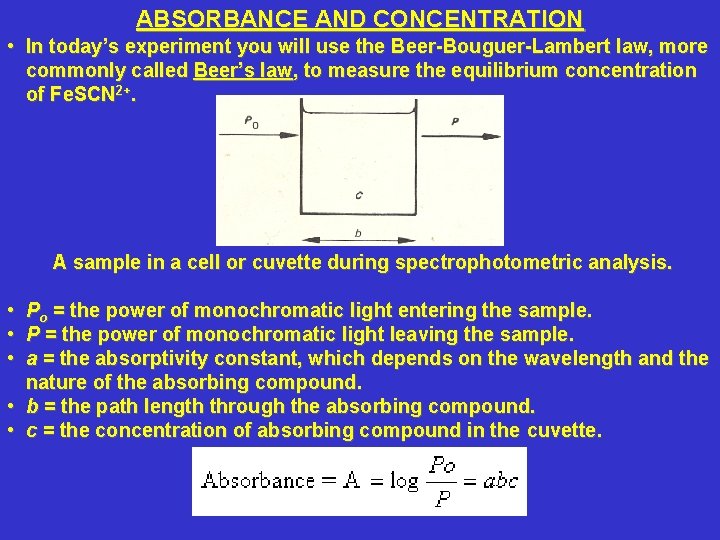
ABSORBANCE AND CONCENTRATION • In today’s experiment you will use the Beer-Bouguer-Lambert law, more commonly called Beer’s law, to measure the equilibrium concentration of Fe. SCN 2+. A sample in a cell or cuvette during spectrophotometric analysis. • Po = the power of monochromatic light entering the sample. • P = the power of monochromatic light leaving the sample. • a = the absorptivity constant, which depends on the wavelength and the nature of the absorbing compound. • b = the path length through the absorbing compound. • c = the concentration of absorbing compound in the cuvette.

SOURCES • Beck, J. 2006. Unit 3 Spectrophotometry. Available: http: //iws. ccccd. edu/jbeck/Spectrophotometryweb/Page. ht ml [accessed 2 October 2006]. • Christian, G. D. 1986. Analytical Chemistry, 3 rd ed. New York, NY: John Wiley & Sons, Inc. • Harris, D. C. 1999. Quantitative Chemical Analysis, 5 th ed. New York, NY: W. H. Freeman Company. • Mc. Murry, J. , R. C. Fay. 2004. Chemistry, 4 th ed. Upper Saddle River, NJ: Prentice Hall. • Petrucci, R. H. 1985. General Chemistry Principles and Modern Applications, 4 th ed. New York, NY: Macmillan Publishing Company.
- Slides: 12