CH 103 SPECTROPHOTOMETRY THE ELECTROMAGNETIC SPECTRUM Electromagnetic radiation








![SOURCES • Aquanic. 2006. Fishkill. Available: http: //aquanic. org/images/photos/illin/fishkill. jpg [accessed 2 September 2006]. SOURCES • Aquanic. 2006. Fishkill. Available: http: //aquanic. org/images/photos/illin/fishkill. jpg [accessed 2 September 2006].](https://slidetodoc.com/presentation_image/989be819ebbb1225eeb54eacd7567395/image-19.jpg)
- Slides: 19

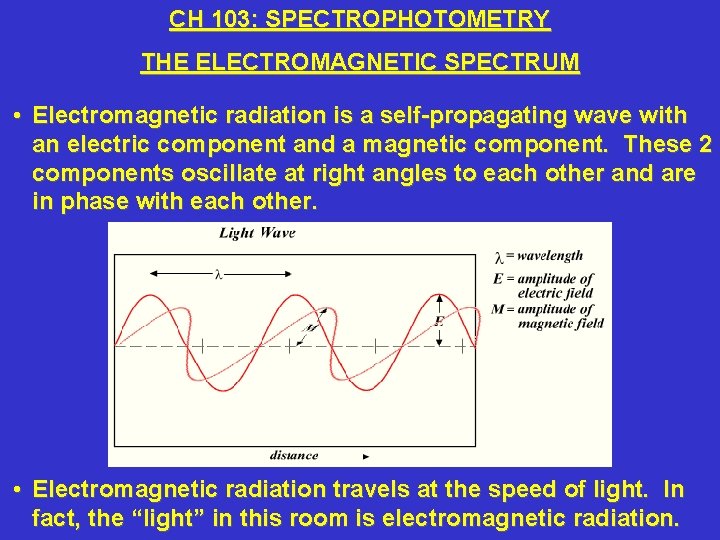
CH 103: SPECTROPHOTOMETRY THE ELECTROMAGNETIC SPECTRUM • Electromagnetic radiation is a self-propagating wave with an electric component and a magnetic component. These 2 components oscillate at right angles to each other and are in phase with each other. • Electromagnetic radiation travels at the speed of light. In fact, the “light” in this room is electromagnetic radiation.

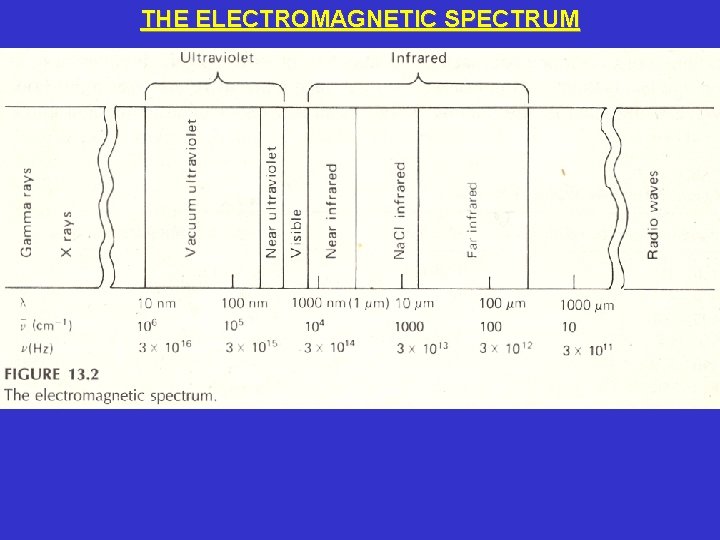
THE ELECTROMAGNETIC SPECTRUM • The wavelength (λ, the length of 1 cycle in meters) times the frequency (ν, the number of cycles per second) equals the speed of light (c, a constant that equals 3. 0 x 108 meters/second). That is, c = λν = 3. 0 x 108 meters/second • If λ increases, then ν must decrease so that c remains constant. • If λ decreases, then ν must increase so that c remains constant.

THE ELECTROMAGNETIC SPECTRUM • Electromagnetic radiation is also a stream of energy packets called photons. • The energy of a single photon (E, in joules) equals Planck’s constant (h, 6. 626 x 10 -34 joule second) times the frequency (ν, the number of cycles per second). That is, E = hν = hc/λ • If the frequency (ν) increases, the energy (E) increases. • If the wavelength (λ) decreases, the energy (E) increases.

THE ELECTROMAGNETIC SPECTRUM

THE ELECTROMAGNETIC SPECTRUM • The ultraviolet (UV) region of the electromagnetic spectrum includes all wavelengths from 10 nanometers (nm) to 380 nm. The vacuum-ultraviolet region goes from 10 nm to 200 nm because air absorbs strongly at these wavelengths so instruments must be operated under a vacuum in this region. The near-ultraviolet region goes from 200 nm to 380 nm. • The visible (Vis) region goes from 380 nm to 780 nm and can be seen by the human eye. • The infrared (IR) region goes from 0. 78 micrometers (μm) or 780 nm to 300 μm. However, the near-infrared (0. 8 μm to 2. 5 μm) and the Na. Cl-infrared regions (2. 5 μm to 16 μm) are the most commonly used by analytical chemists.

THE ABSORPTION OF ELECTROMAGNETIC RADIATION BY MOLECULES • Humans see color when an object transmits or reflects visible light. • More specifically, an object may absorb specific wavelengths of electromagnetic radiation. The unabsorbed wavelengths from the visible region are transmitted and seen as color. • For example, leaves are green because the pigment chlorophyll absorbs violet, blue, and red light. • Why is my car blue? • It’s blue because it absorbs yellow.

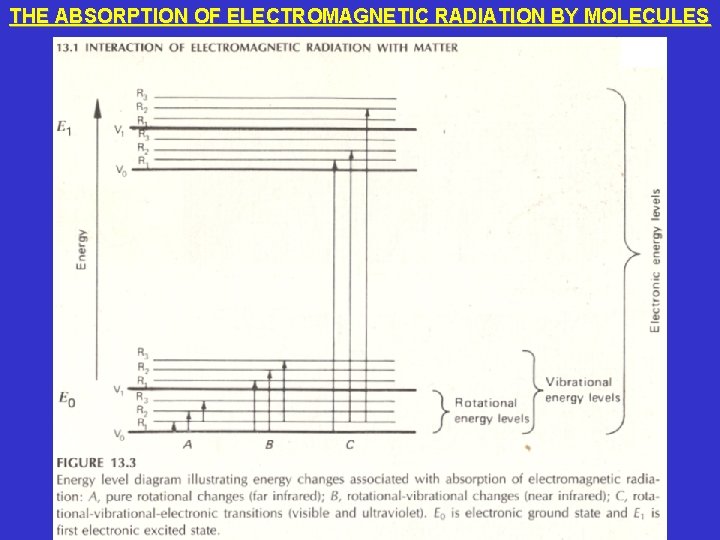
THE ABSORPTION OF ELECTROMAGNETIC RADIATION BY MOLECULES • There are 3 ways that a molecule can absorb electromagnetic radiation. All 3 ways raise the molecule to a higher internal energy level. All these changes in energy are quantized; that is, they occur at discrete levels. • Rotational Transitions: The molecule rotates around various axes. Rotational transitions require the least amount of energy. Purely rotational transitions can occur in the far-infrared and microwave regions. • Vibrational Transitions: Atoms or groups of atoms within a molecule vibrate relative to each other. Vibrational transitions require an intermediate amount of energy and typically begin to occur in the midinfrared and far-infrared regions. Therefore, as energy is increased (or wavelength is decreased) vibrational transitions occur in addition to rotational transitions. • Electronic Transitions: An electron within a molecule is typically promoted from its ground state to an excited state. Electronic transitions require the most amount of energy and typically begin to occur in the visible and ultraviolet regions. Therefore, as energy is increased (or wavelength is decreased) electronic transitions occur in addition to vibrational and rotational transitions.

THE ABSORPTION OF ELECTROMAGNETIC RADIATION BY MOLECULES

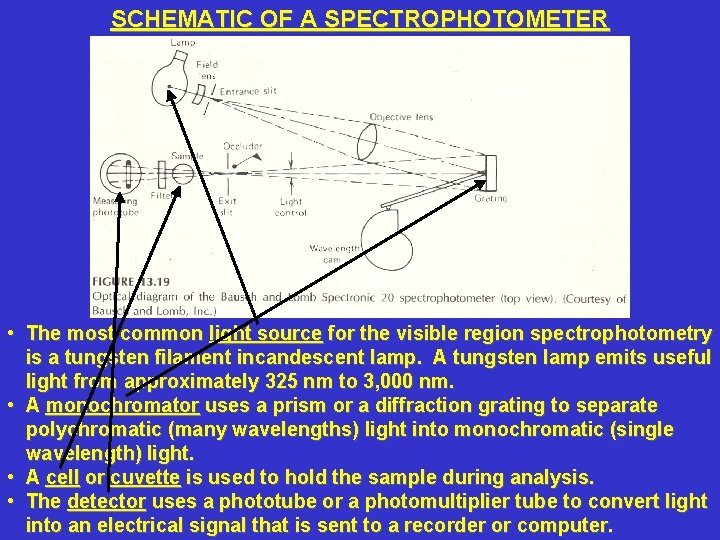
SCHEMATIC OF A SPECTROPHOTOMETER • The most common light source for the visible region spectrophotometry is a tungsten filament incandescent lamp. A tungsten lamp emits useful light from approximately 325 nm to 3, 000 nm. • A monochromator uses a prism or a diffraction grating to separate polychromatic (many wavelengths) light into monochromatic (single wavelength) light. • A cell or cuvette is used to hold the sample during analysis. • The detector uses a phototube or a photomultiplier tube to convert light into an electrical signal that is sent to a recorder or computer.

THE SPECTRONIC 20 D SPECTROPHOTOMETER The controls. Loading a sample. THE HACH DR 2010 SPECTROPHOTOMETER

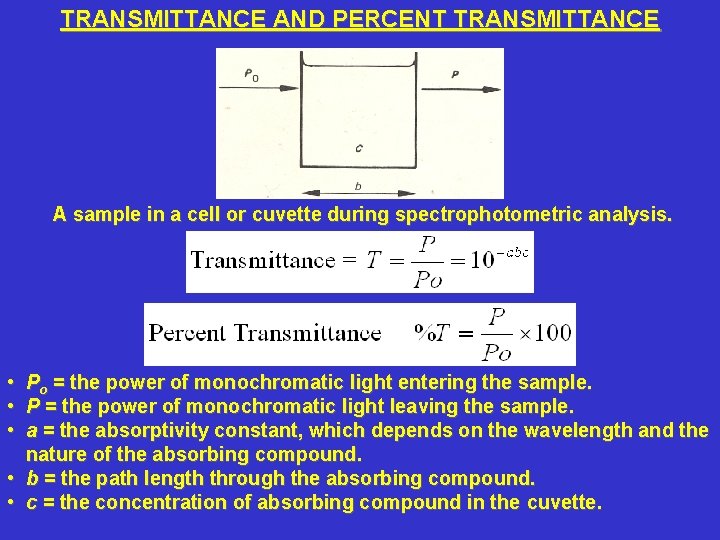
TRANSMITTANCE AND PERCENT TRANSMITTANCE A sample in a cell or cuvette during spectrophotometric analysis. • Po = the power of monochromatic light entering the sample. • P = the power of monochromatic light leaving the sample. • a = the absorptivity constant, which depends on the wavelength and the nature of the absorbing compound. • b = the path length through the absorbing compound. • c = the concentration of absorbing compound in the cuvette.

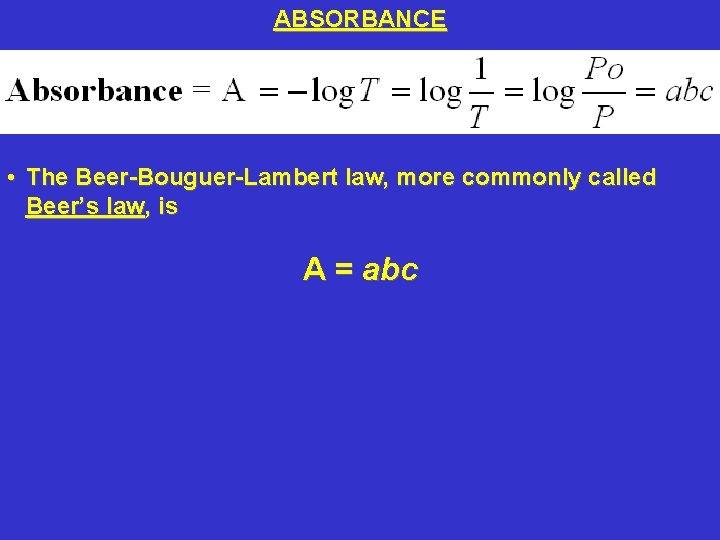
ABSORBANCE • The Beer-Bouguer-Lambert law, more commonly called Beer’s law, is A = abc

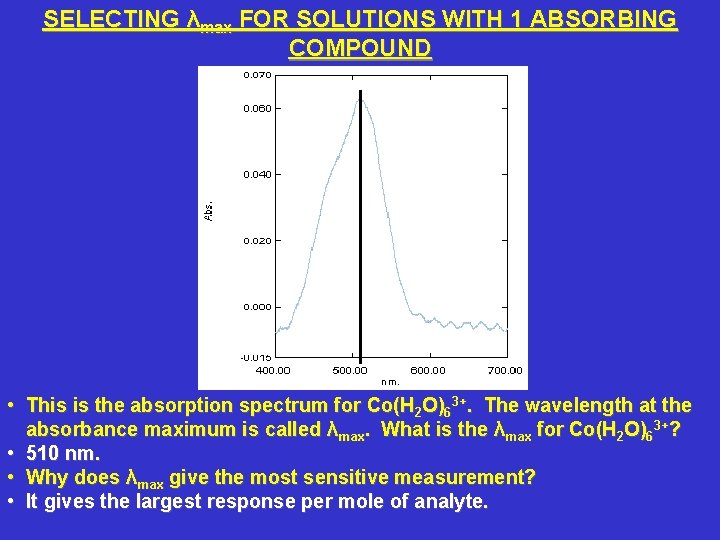
SELECTING λmax FOR SOLUTIONS WITH 1 ABSORBING COMPOUND • This is the absorption spectrum for Co(H 2 O)63+. The wavelength at the absorbance maximum is called λmax. What is the λmax for Co(H 2 O)63+? • 510 nm. • Why does λmax give the most sensitive measurement? • It gives the largest response per mole of analyte.

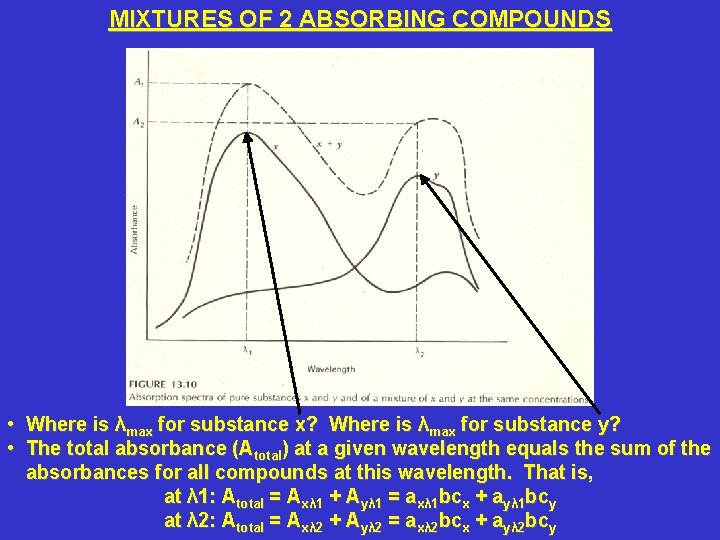
MIXTURES OF 2 ABSORBING COMPOUNDS • Where is λmax for substance x? Where is λmax for substance y? • The total absorbance (Atotal) at a given wavelength equals the sum of the absorbances for all compounds at this wavelength. That is, at λ 1: Atotal = Axλ 1 + Ayλ 1 = axλ 1 bcx + ayλ 1 bcy at λ 2: Atotal = Axλ 2 + Ayλ 2 = axλ 2 bcx + ayλ 2 bcy

CASE STUDY: UV/Vis SPECTROSCOPY AND THE FOX RIVER MYSTERY • In 1988 over 30, 000 fish died suddenly and unexpectedly in the Fox River at Oshkosh, Wisconsin. Such “fish kills” are often caused by a lack of dissolved oxygen (O 2), or a release of pesticides, organic compounds, chlorine (Cl 2), or heavy metals into the environment. However, none of these caused the Fox River fish kill.

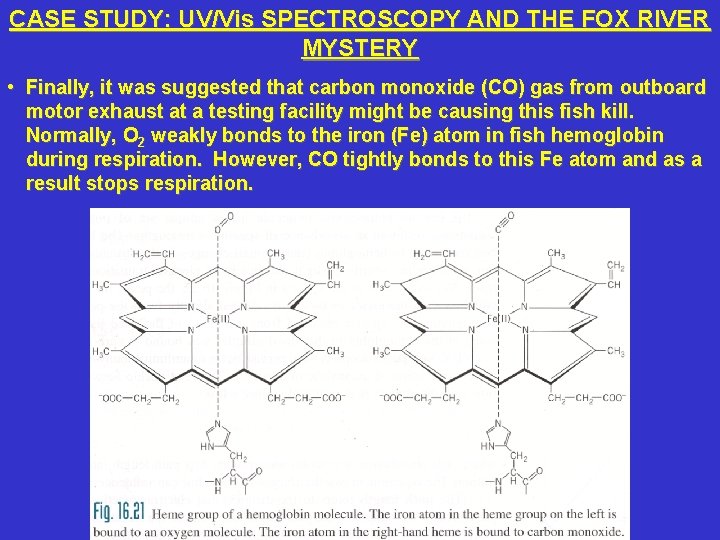
CASE STUDY: UV/Vis SPECTROSCOPY AND THE FOX RIVER MYSTERY • Finally, it was suggested that carbon monoxide (CO) gas from outboard motor exhaust at a testing facility might be causing this fish kill. Normally, O 2 weakly bonds to the iron (Fe) atom in fish hemoglobin during respiration. However, CO tightly bonds to this Fe atom and as a result stops respiration.

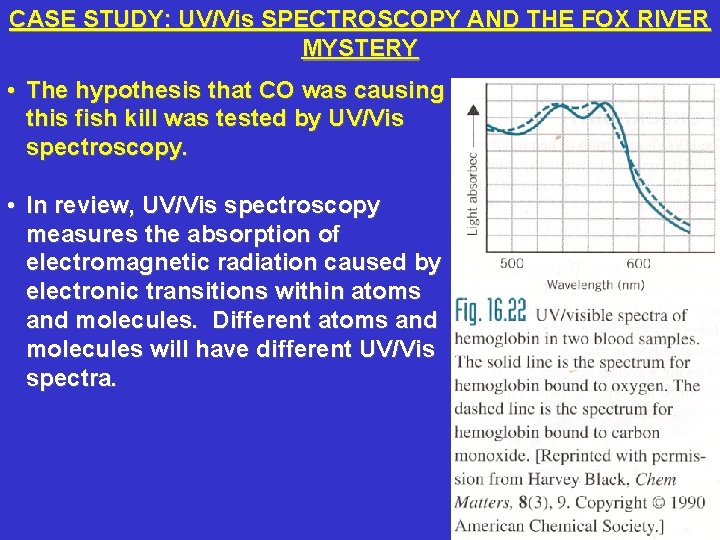
CASE STUDY: UV/Vis SPECTROSCOPY AND THE FOX RIVER MYSTERY • The hypothesis that CO was causing this fish kill was tested by UV/Vis spectroscopy. • In review, UV/Vis spectroscopy measures the absorption of electromagnetic radiation caused by electronic transitions within atoms and molecules. Different atoms and molecules will have different UV/Vis spectra.

TODAY’S EXPERIMENT • Work in groups of 4. • Every student does 1 quantitative analysis. • Every student in each group gets a different color (blue, purple, red, or yellow) of crepe paper. • Each student selects λmax from an absorption spectrum of their crepe paper. • Each student makes 4 standard solutions by extracting different amounts of dye from their crepe paper. • Each student uses these solutions and Beer’s law to make a calibration curve. • Each student uses this calibration curve to measure the concentration of an unknown solution that was made from their assigned color of crepe paper. • Every group does 1 qualitative analysis. • Each group analyses the absorption spectrum from another unknown solution that was made by extracting the dye from 2 different colors of crepe paper. They will determine which 2 colors were used to make this solution.
![SOURCES Aquanic 2006 Fishkill Available http aquanic orgimagesphotosillinfishkill jpg accessed 2 September 2006 SOURCES • Aquanic. 2006. Fishkill. Available: http: //aquanic. org/images/photos/illin/fishkill. jpg [accessed 2 September 2006].](https://slidetodoc.com/presentation_image/989be819ebbb1225eeb54eacd7567395/image-19.jpg)
SOURCES • Aquanic. 2006. Fishkill. Available: http: //aquanic. org/images/photos/illin/fishkill. jpg [accessed 2 September 2006]. • Beck, J. 2006. Unit 3 Spectrophotometry. Available: http: //iws. ccccd. edu/jbeck/Spectrophotometryweb/Page. html [accessed 2 October 2006]. • Christian, G. D. 1986. Analytical Chemistry, 3 rd ed. New York, NY: John Wiley & Sons, Inc. • Harris, D. C. 1999. Quantitative Chemical Analysis, 5 th ed. New York, NY: W. H. Freeman Company. • Raven, P. H. , R. F. Evert, H. Curtis. 1981. Biology of Plants, 3 rd ed. New York, NY: Worth Publishers, Inc. • Spencer, J. N. , G. M. Bodner, L. H. Rickard. 2006. Chemistry Structure and Dynamics, 3 rd ed. New York, NY: John Wiley & Sons, Inc. • Wikipedia. 2006. Image: Light-wave. png. Available: http: //en. wikipedia. org/wiki/Image: Light-wave. png [accessed 2 September 2006].