CH 103 ATOMIC MASS AND ISOTOPIC ABUNDANCE GAS

CH 103: ATOMIC MASS AND ISOTOPIC ABUNDANCE GAS CHROMATOGRAPHY MASS SPECTROMETRY • Gas chromatography = separation • Mass spectrometry = detection
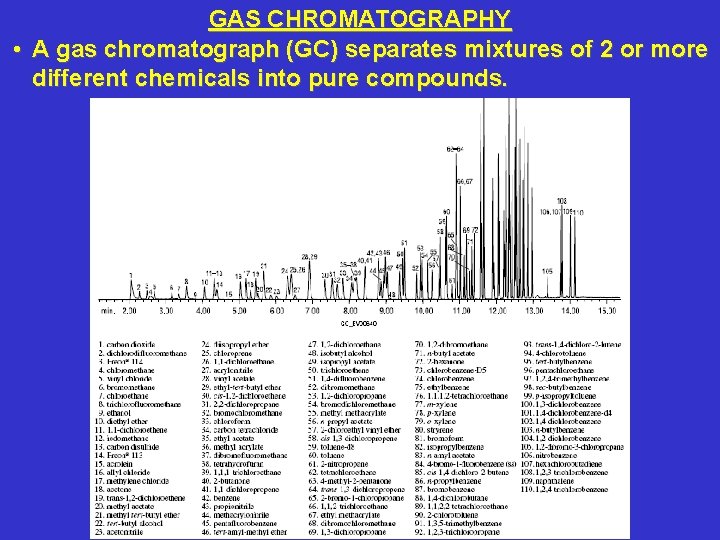
GAS CHROMATOGRAPHY • A gas chromatograph (GC) separates mixtures of 2 or more different chemicals into pure compounds.

GAS CHROMATOGRAPHY • The sample is injected into the GC inlet where it is heated and swept onto a chromatographic column by a carrier gas. • The pure compounds in a mixture are separated by interacting with the coating or packing of the column (stationary phase) and the carrier gas (mobile phase). • This separation is often improved by programming changes in column temperature and pressure.

GAS CHROMATOGRAPHY Splitless Injection Animation http: //www. restek. com/info_animation_splitless. asp • Splitless injections direct the entire sample to the column. 2 m. L/min septum purge flow + 3 m. L/min column flow = 5 m. L/min total flow • Unknowns might be analyzed with a 0. 2 μL splitless injection to identify minor components.

GAS CHROMATOGRAPHY Split Injection Animation http: //www. restek. com/info_animation_split. asp • Split injections direct a fraction of the sample to the column and the remaining sample vents out of the GC. 2 m. L/min septum purge flow + 1 m. L/min column flow + 100 m. L/min split flow = 103 m. L/min total flow • Complicated mixtures, such as petroleum, might be analyzed with a 1. 0 μL injection using a 1 to 100 split to identify major components.
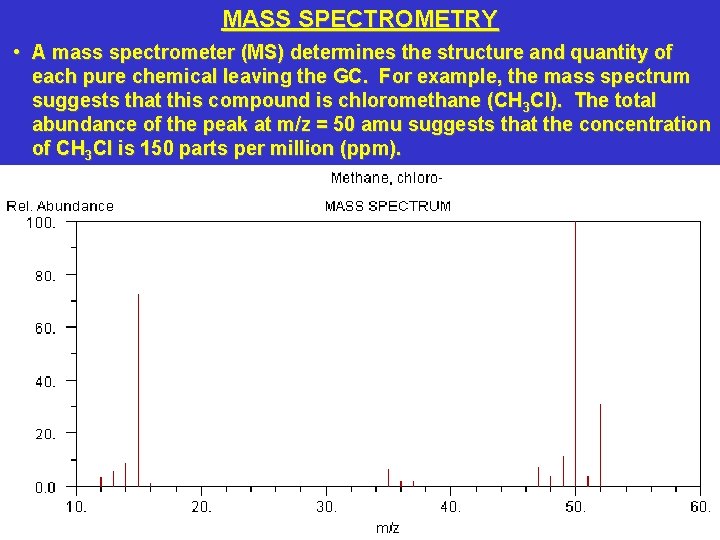
MASS SPECTROMETRY • A mass spectrometer (MS) determines the structure and quantity of each pure chemical leaving the GC. For example, the mass spectrum suggests that this compound is chloromethane (CH 3 Cl). The total abundance of the peak at m/z = 50 amu suggests that the concentration of CH 3 Cl is 150 parts per million (ppm).
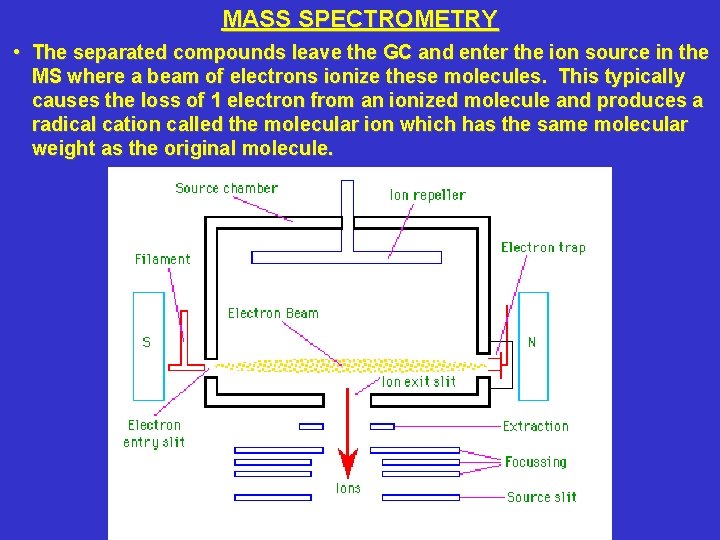
MASS SPECTROMETRY • The separated compounds leave the GC and enter the ion source in the MS where a beam of electrons ionize these molecules. This typically causes the loss of 1 electron from an ionized molecule and produces a radical cation called the molecular ion which has the same molecular weight as the original molecule.
MASS SPECTROMETRY
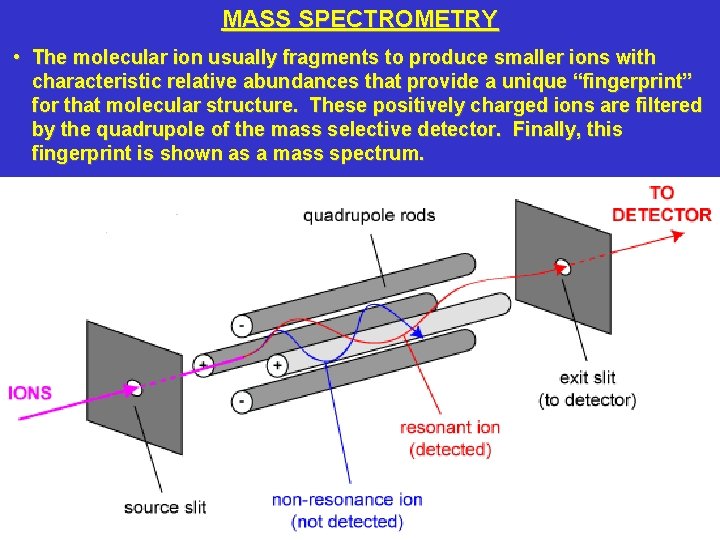
MASS SPECTROMETRY • The molecular ion usually fragments to produce smaller ions with characteristic relative abundances that provide a unique “fingerprint” for that molecular structure. These positively charged ions are filtered by the quadrupole of the mass selective detector. Finally, this fingerprint is shown as a mass spectrum.
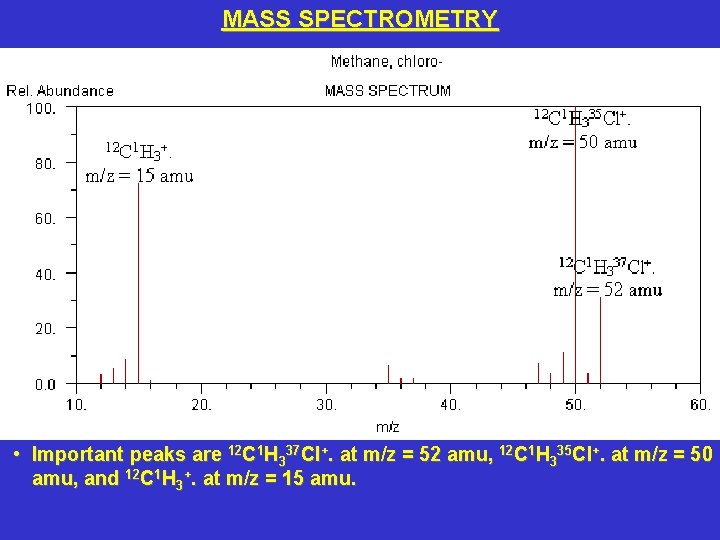
MASS SPECTROMETRY • Important peaks are 12 C 1 H 337 Cl+. at m/z = 52 amu, 12 C 1 H 335 Cl+. at m/z = 50 amu, and 12 C 1 H 3+. at m/z = 15 amu.
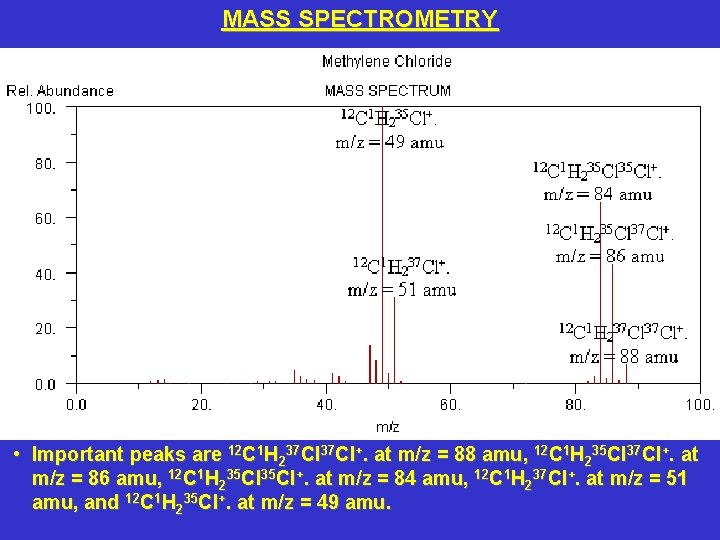
MASS SPECTROMETRY • Important peaks are 12 C 1 H 237 Cl+. at m/z = 88 amu, 12 C 1 H 235 Cl 37 Cl+. at m/z = 86 amu, 12 C 1 H 235 Cl+. at m/z = 84 amu, 12 C 1 H 237 Cl+. at m/z = 51 amu, and 12 C 1 H 235 Cl+. at m/z = 49 amu.
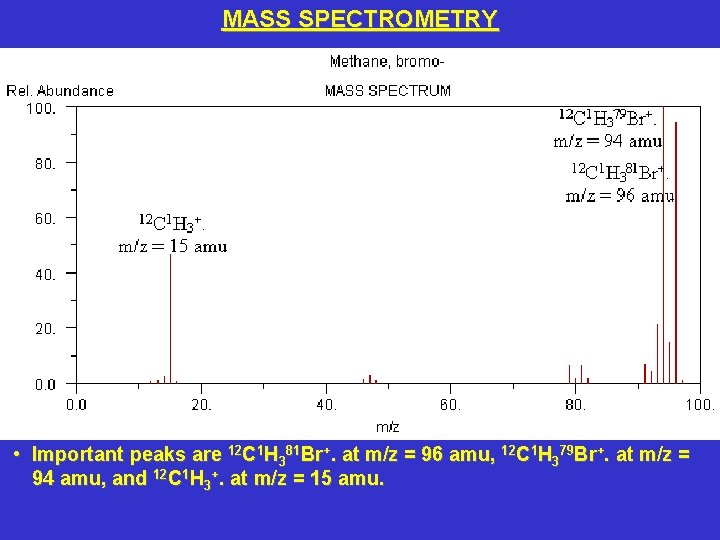
MASS SPECTROMETRY • Important peaks are 12 C 1 H 381 Br+. at m/z = 96 amu, 12 C 1 H 379 Br+. at m/z = 94 amu, and 12 C 1 H 3+. at m/z = 15 amu.
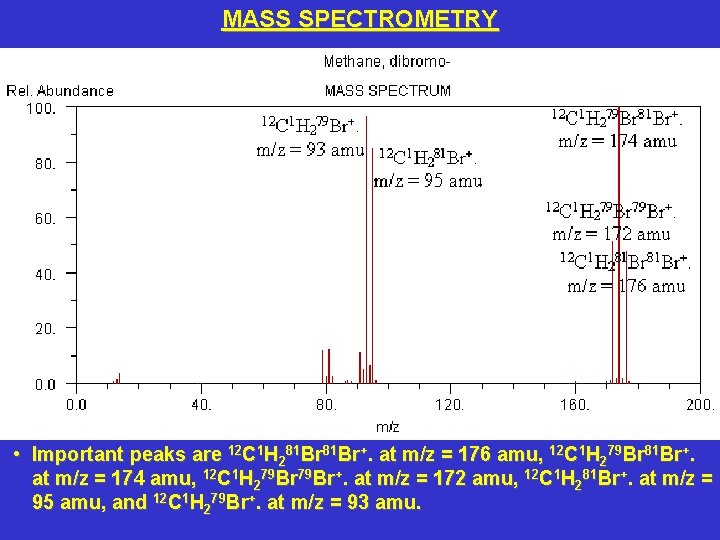
MASS SPECTROMETRY • Important peaks are 12 C 1 H 281 Br+. at m/z = 176 amu, 12 C 1 H 279 Br 81 Br+. at m/z = 174 amu, 12 C 1 H 279 Br+. at m/z = 172 amu, 12 C 1 H 281 Br+. at m/z = 95 amu, and 12 C 1 H 279 Br+. at m/z = 93 amu.
LIQUID CHROMATOGRAPHY MASS SPECTROMETRY Liquid Chromatography Mass Spectrometry Animation http: //www. chem. agilent. com/Scripts/Generic. ASP? l. Page=10184&indcol=N&prodcol=Y

SOURCES • Agilent Technologies. 2006. Agilent. Available: http: //www. home. agilent. com/agilent/home. jspx? cc=US&lc=eng&cmpid=4533 [accessed 1 September 2006]. • Agilent Technologies. 2006. 6210 Time-of-Flight LC/MS. Available: http: //www. chem. agilent. com/Scripts/Generic. ASP? l. Page=10184&indcol=N&pro dcol=Y [accessed 1 September 2006]. • Bull I. D. , P. Gates. 2005. Gas Chromatography Mass Spectrometry (GC/MS). Available: http: //www. bris. ac. uk/nerclsmsf/techniques/gcms. html [accessed 1 September 2006]. • Mc. Lafferty, F. W. 1980. Interpretation of Mass Spectra, 3 rd ed. Mill Valley, CA: University Science Books. • National Institute of Standards and Technology. 2006. NIST Chemistry Web. Book. Available: http: //webbook. nist. gov/chemistry/ [accessed 1 September 2006]. • Restek Corporation. 2006. Split Injection Animation. Available: http: //www. restek. com/info_animation_split. asp [accessed 1 September 2006]. • Restek Corporation. 2006. Splitless Injection Animation. Available: http: //www. restek. com/info_animation_splitless. asp [accessed 1 September 2006].
- Slides: 15