Ch 10 Photosynthesis Organisms need organic compounds for







- Slides: 25

Ch 10 Photosynthesis

Organisms need organic compounds for: energy and carbon skeletons Two types of organisms: heterotrophs – take in organic compounds from other organisms autotrophs – make their own organic comp. Two types of autotrophs (“self-feeders”): • Chemoautotrophs: obtain energy by oxidizing inorganic compounds w/out light (rare, bacteria). • Photoautotrophs: use light energy to produce organic compounds. • Photoautotrophs include: plants, algae, some protists • Humans rely on photoautotrophs for: food & oxygen

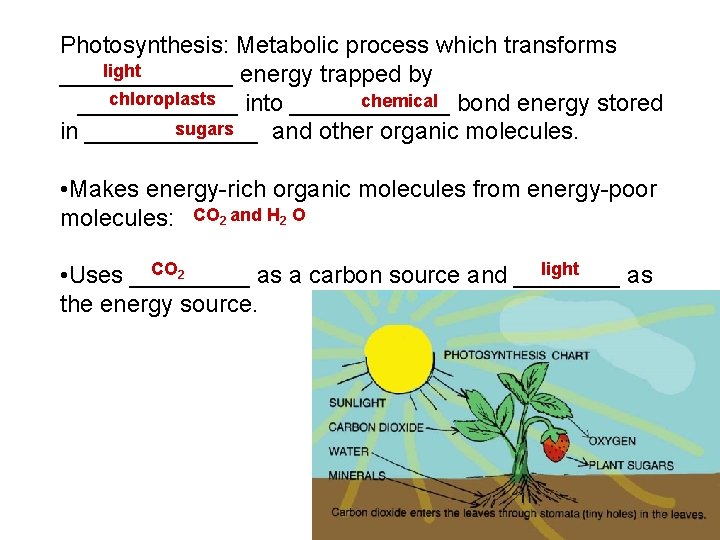
Photosynthesis: Metabolic process which transforms light _______ energy trapped by chloroplasts chemical bond energy stored ______ into ______ sugars in _______ and other organic molecules. • Makes energy-rich organic molecules from energy-poor molecules: CO 2 and H 2 O CO 2 light • Uses _____ as a carbon source and ____ as the energy source.

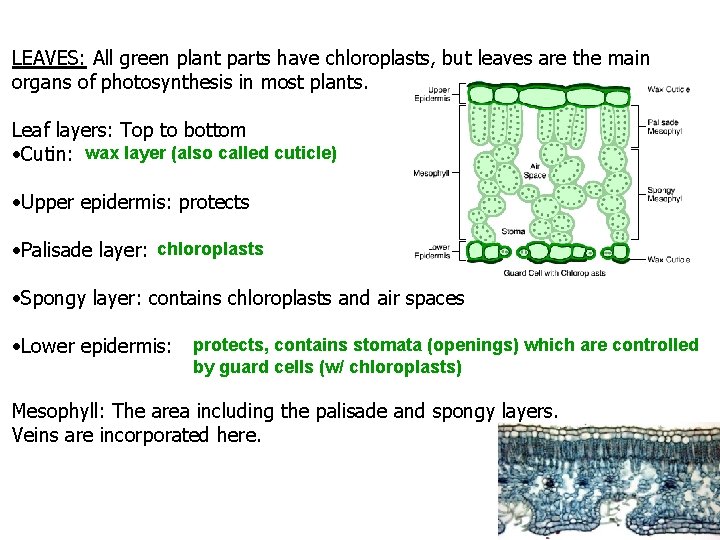
LEAVES: All green plant parts have chloroplasts, but leaves are the main organs of photosynthesis in most plants. Leaf layers: Top to bottom Cutin: wax layer (also called cuticle) Upper epidermis: protects Palisade layer: chloroplasts Spongy layer: contains chloroplasts and air spaces Lower epidermis: protects, contains stomata (openings) which are controlled by guard cells (w/ chloroplasts) Mesophyll: The area including the palisade and spongy layers. Veins are incorporated here.

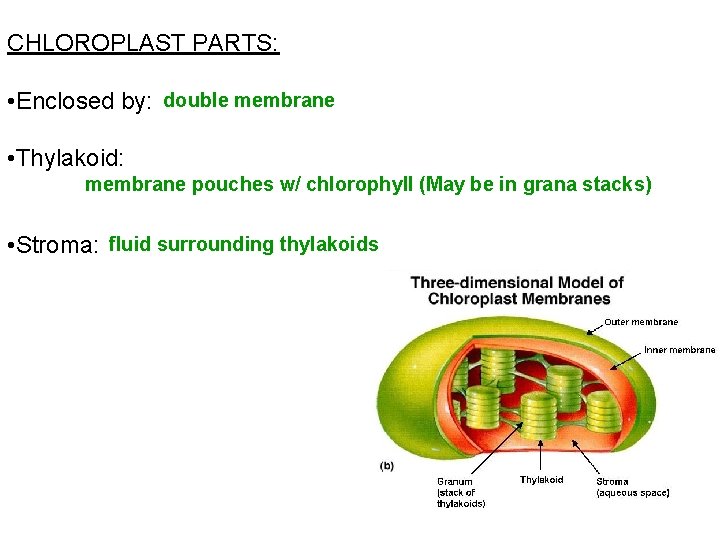
CHLOROPLAST PARTS: • Enclosed by: double membrane • Thylakoid: membrane pouches w/ chlorophyll (May be in grana stacks) • Stroma: fluid surrounding thylakoids

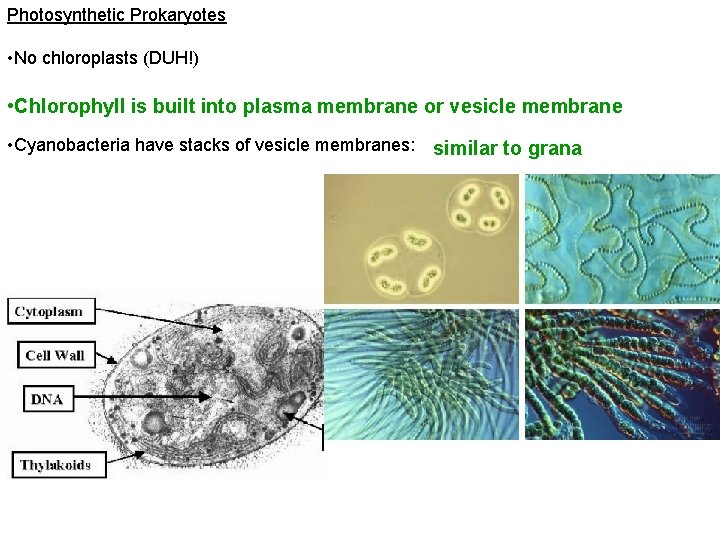
Photosynthetic Prokaryotes • No chloroplasts (DUH!) • Chlorophyll is built into plasma membrane or vesicle membrane • Cyanobacteria have stacks of vesicle membranes: similar to grana

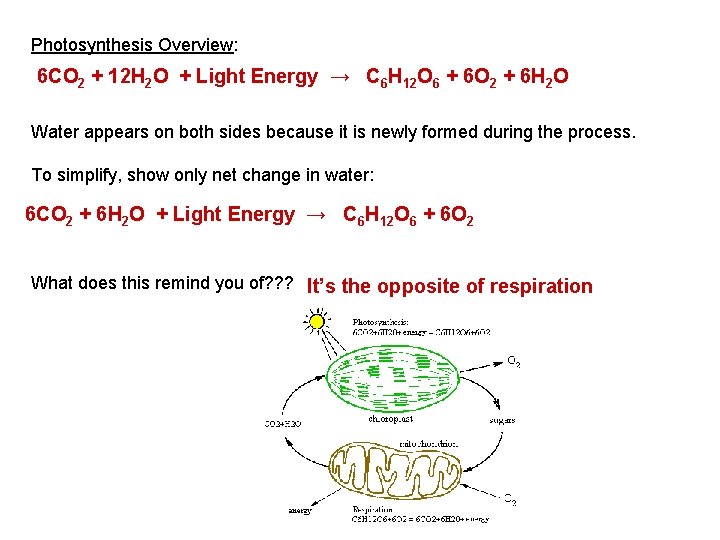
Photosynthesis Overview: 6 CO 2 + 12 H 2 O + Light Energy → C 6 H 12 O 6 + 6 O 2 + 6 H 2 O Water appears on both sides because it is newly formed during the process. To simplify, show only net change in water: 6 CO 2 + 6 H 2 O + Light Energy → C 6 H 12 O 6 + 6 O 2 What does this remind you of? ? ? It’s the opposite of respiration

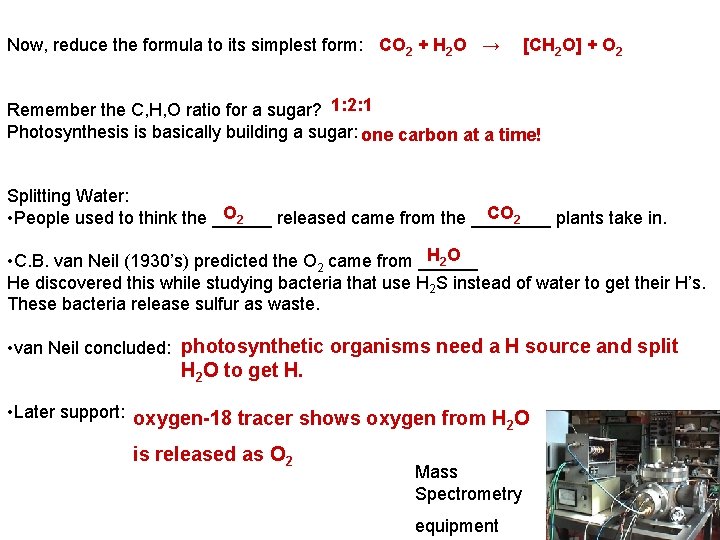
Now, reduce the formula to its simplest form: CO 2 + H 2 O → [CH 2 O] + O 2 Remember the C, H, O ratio for a sugar? 1: 2: 1 Photosynthesis is basically building a sugar: one carbon at a time! Splitting Water: O 2 CO 2 • People used to think the ______ released came from the ____ plants take in. H 2 O • C. B. van Neil (1930’s) predicted the O 2 came from ______ He discovered this while studying bacteria that use H 2 S instead of water to get their H’s. These bacteria release sulfur as waste. • van Neil concluded: photosynthetic organisms need a H source and split H 2 O to get H. • Later support: oxygen-18 tracer shows oxygen from H O 2 is released as O 2 Mass Spectrometry equipment

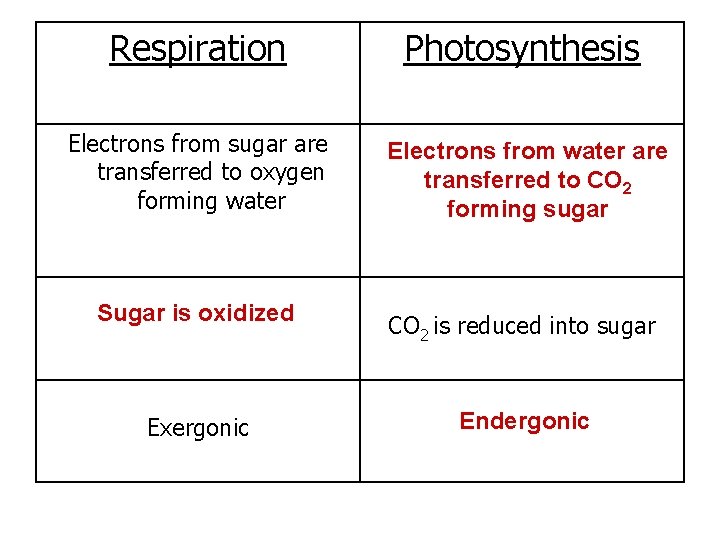
Respiration Photosynthesis Electrons from sugar are transferred to oxygen forming water Electrons from water are transferred to CO 2 forming sugar Sugar is oxidized CO 2 is reduced into sugar Exergonic Endergonic

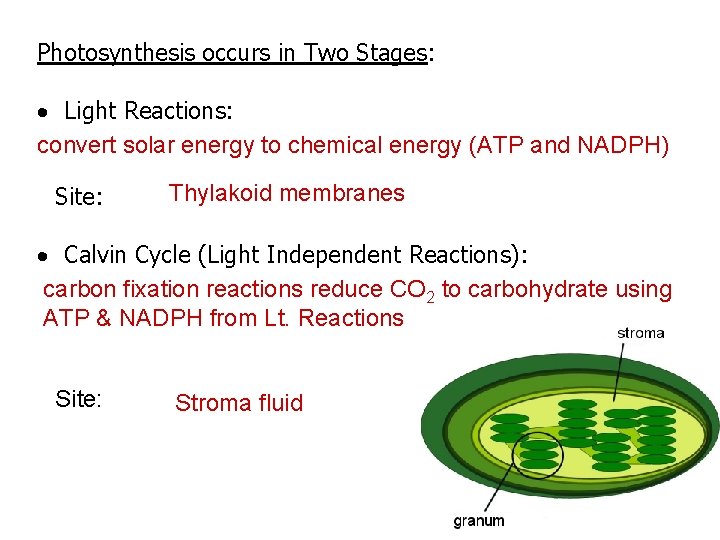
Photosynthesis occurs in Two Stages: Light Reactions: convert solar energy to chemical energy (ATP and NADPH) Site: Thylakoid membranes Calvin Cycle (Light Independent Reactions): carbon fixation reactions reduce CO 2 to carbohydrate using ATP & NADPH from Lt. Reactions Site: Stroma fluid

Sunlight: Electromagnetic energy (radiation) has a behavior that is both wavelike and particlelike. WAVELIKE PROPERTIES: rhythmic disturbances of electric and magnetic fields crests • Wavelength = distance between _______ of waves. These range from <1 nm to >1 km for the Electromagnetic Spectrum. • Visible Light = 380 – 750 nm wavelength: The portion of the Elec. Spectrum which humans can see (ROYGBIV) PARTICLELIKE PROPERTIES: • Light also behaves as if it consists of particles called: photons (or quanta) Photon = fixed quantity of energy (inversely proportional to wavelength) more • Shorter wavelengths = ________ energy (violet more energy than red)

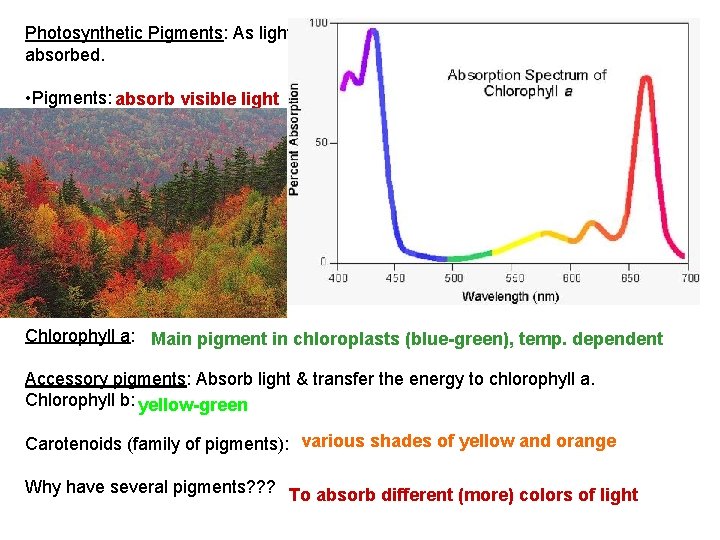
Photosynthetic Pigments: As light meets matter, it can be reflected, transmitted, or absorbed. • Pigments: absorb visible light • Different pigments absorb light of different wavelengths: each pigment has an “absorption spectrum” • The wavelengths that are absorbed: “disappear. ” The colors we see are those reflected! • Spectrophotometer: measures the ability of a pigment to absorb various wavelengths. Chlorophyll a: Main pigment in chloroplasts (blue-green), temp. dependent Accessory pigments: Absorb light & transfer the energy to chlorophyll a. Chlorophyll b: yellow-green Carotenoids (family of pigments): various shades of yellow and orange Why have several pigments? ? ? To absorb different (more) colors of light

Photooxidation of Chlorophyll When pigments absorb photons: • Colors of absorbed light disappear from spectrum, but energy cannot disappear. electrons from its lowest energy • Photon boosts one of the pigment molecule’s ______ (ground state) state ____________ to an orbital of higher potential energy (excited state) _____________ • Excited state is unstable: Without intervention, electron would fall back and release energy (as heat or fluorescence) • Thylakoid membranes contain electron acceptors which trap the excited electrons before they can return to ground state. oxidized reduced • Chlorophyll is _______ while the electron acceptor is ________.

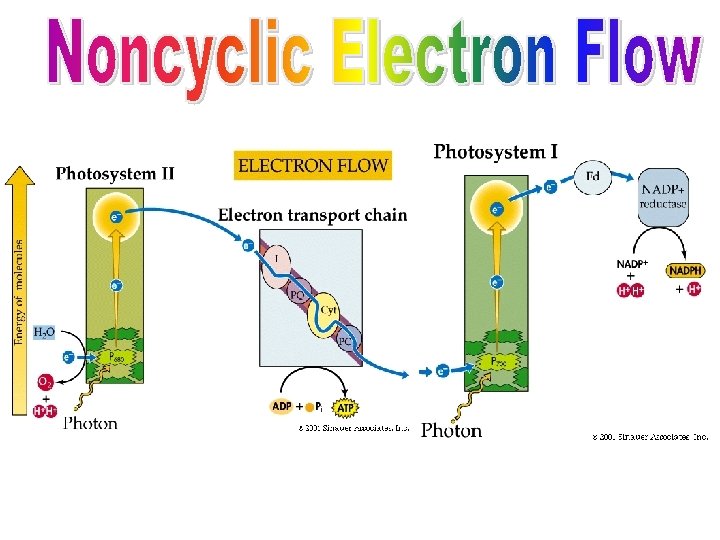
Photosystem Assembly: In thylakoid, 3 parts: • Antenna complex = 100’s of pigment molecules • Reaction center = only pair of chlorophyll a molecules which can donate e- to the eacceptor • Primary e- acceptor TWO TYPES OF PHOTOSYSTEMS: Differ in their location relative to specific proteins and e- acceptors. • Photosystem I: Absorbs far red (700 nm) light best • Photosystem II: Absorbs red best (680 nm) Two routes for Electron Flow: Once excited, electrons flow: • Cyclic: Photosystem I • Non-Cyclic: Photosystems I and II

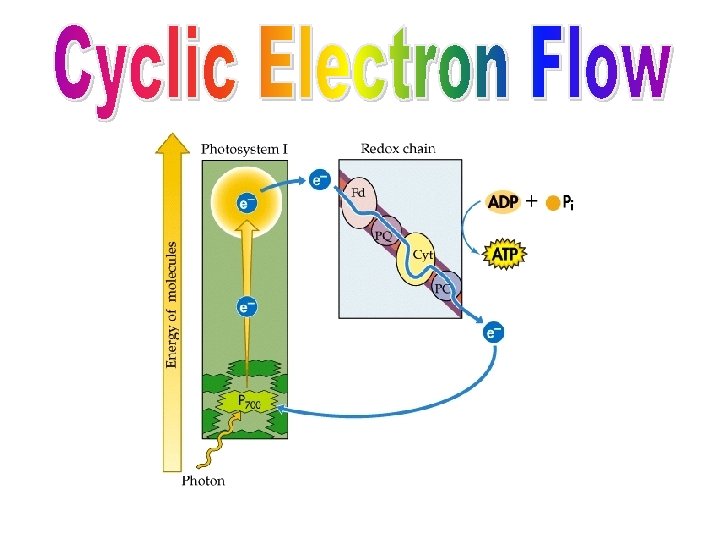
During the light reactions, there are two possible routes for electron flow: cyclic & non-cyclic Cyclic Electron Flow: • Simpler pathway, involves only photosystem I: generates only ATP (no O 2 or NADPH) • Pigments absorb energy and channel it to: the P 700 reaction center • P 700 chlorophyll a’s electrons become excited, leave the molecule and are trapped by: primary e- acceptor • Electrons are passed along an ETC until returned to their ground state in P 700: They CYCLE back to their start point. • What happens to the energy released by ETC? Pumps H+ ions (into thylakoid from stroma) creates proton-motive force H+ flow through ATP synthase in thylakoid membrane ATP is made • This type of ATP production is called: cyclic photophosphorylation


Non. Cyclic Electron Flow: • Involves both photosystem I and photosystem II • (SAME) Pigments absorb energy and channel it to: the P 700 reaction center • (SAME) P 700 chlorophyll a’s electrons become excited, leave the molecule and are trapped by: primary e- acceptor • Electrons are passed to NADP+ with H+ from water = NADPH (High energy e-) • The e- that left the chlorophyll a must be replaced: photosystem II does it • Light energy absorbed by P 680 excites e- which are passed to the same ETC as cyclic electron flow until they reach P 700 and replace the missing electrons: the ETC makes ATP (same as cyclic flow) • The actual ATP production is the same as cyclic, but is called: noncyclic photophosphorylation • The P 680 e- are replaced by e- from: water • WATER was split into: H+ (joined NADP+) e- (replaces P 680 e-) O (joins another O to be released as O 2)


Cyclic and Non-cyclic Electron Flow: Why have both? ? ? ATP NADPH noncyclic Cavin cycle requires more ____ than ______ and ________ equal ATP flow makes roughly _____ amounts. Cyclic flow makes the extra ______ needed. ATP Synthesis: Respiration vs. Photosynthesis • Both use chemiosmosis • ATP synthase and many e- carriers are similar. • Energy source is different: food vs. light • Proton gradient (p. H gradient): experiments from photosynthesis support chemiosmosis occurs in both

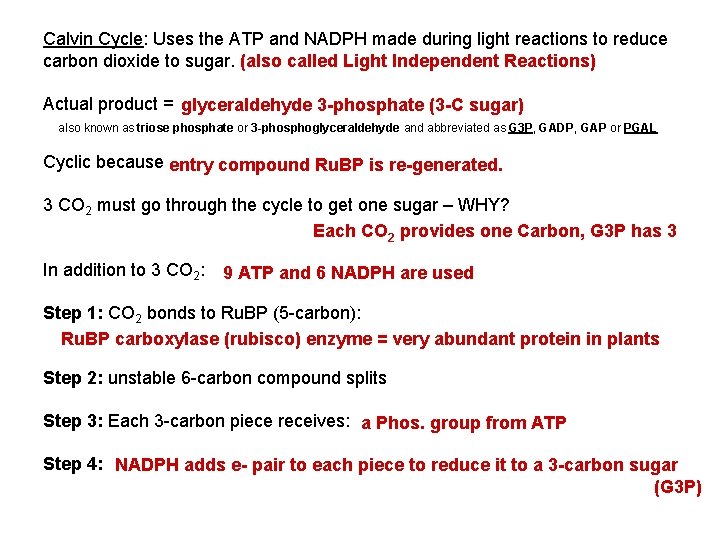
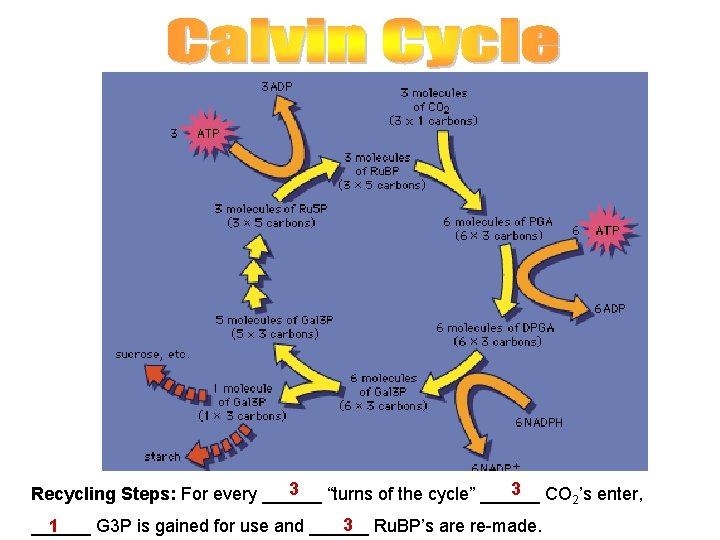
Calvin Cycle: Uses the ATP and NADPH made during light reactions to reduce carbon dioxide to sugar. (also called Light Independent Reactions) Actual product = glyceraldehyde 3 -phosphate (3 -C sugar) also known as triose phosphate or 3 -phosphoglyceraldehyde and abbreviated as G 3 P, GADP, GAP or PGAL Cyclic because entry compound Ru. BP is re-generated. 3 CO 2 must go through the cycle to get one sugar – WHY? Each CO 2 provides one Carbon, G 3 P has 3 In addition to 3 CO 2: 9 ATP and 6 NADPH are used Step 1: CO 2 bonds to Ru. BP (5 -carbon): Ru. BP carboxylase (rubisco) enzyme = very abundant protein in plants Step 2: unstable 6 -carbon compound splits Step 3: Each 3 -carbon piece receives: a Phos. group from ATP Step 4: NADPH adds e- pair to each piece to reduce it to a 3 -carbon sugar (G 3 P)

3 3 CO ’s enter, Recycling Steps: For every ______ “turns of the cycle” ______ 2 3 Ru. BP’s are re-made. ______ G 3 P is gained for use and ______ 1

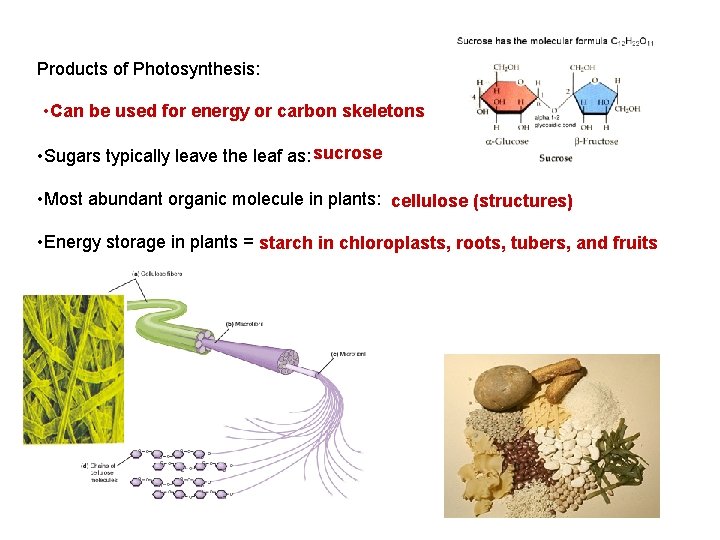
Products of Photosynthesis: • Can be used for energy or carbon skeletons • Sugars typically leave the leaf as: sucrose • Most abundant organic molecule in plants: cellulose (structures) • Energy storage in plants = starch in chloroplasts, roots, tubers, and fruits

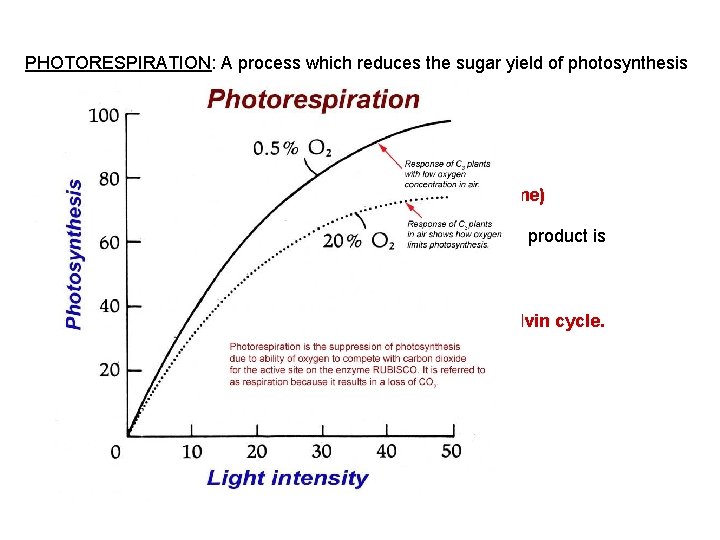
PHOTORESPIRATION: A process which reduces the sugar yield of photosynthesis On hot, dry days: plants close stomata to conserve water Oxygen builds up in leaves and CO 2 decreases. O 2 competes with CO 2 for the active site on Rubisco (enzyme) O 2 CO 2 When ______ is bonded in place of _____, the 2 -carbon product is CO 2 H 2 O (in peroxisomes) oxidized to _______ and ____ This is bad because organic material is taken out of the Calvin cycle. Believed to be: evolutionary relic (no O 2 on early Earth)

Methods of Minimizing Photorespiration: C 4 Plants: Unlike typical (C 3) Plants, C 4 Plants use PEP: PEP binds CO 2 in mesophyll (forms malate intermediate) and shuttles it to rubisco. PEP cannot bond to O 2

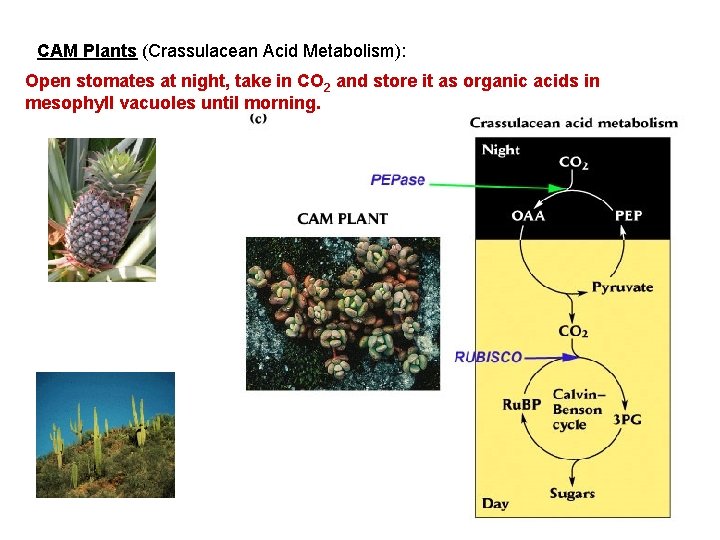
CAM Plants (Crassulacean Acid Metabolism): Open stomates at night, take in CO 2 and store it as organic acids in mesophyll vacuoles until morning.