Ch 10 Kinetics and Equilibrium Kinetics 1 Reaction
Ch. 10 Kinetics and Equilibrium

Ι. Kinetics动力学

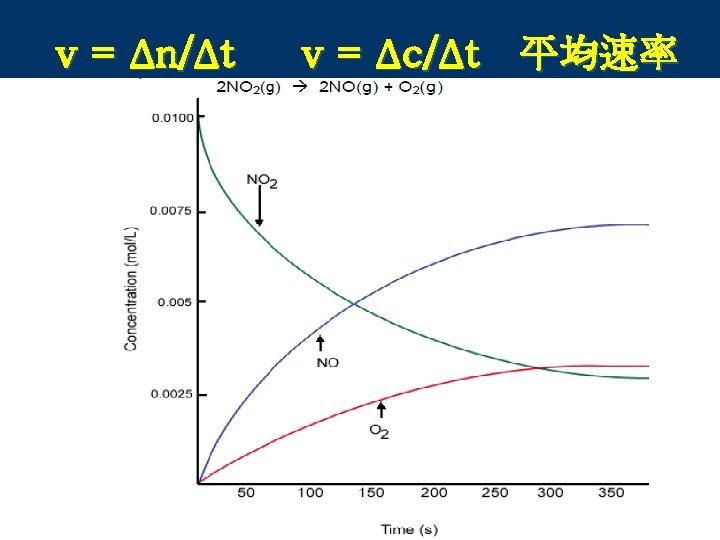
1. Reaction Rate反应速率 n The reaction rate is determined experimentally by measuring the rate at which a reactant disappears or a product appears. So reaction rates are generally measured in moles/time or M/time.

Reaction Rate
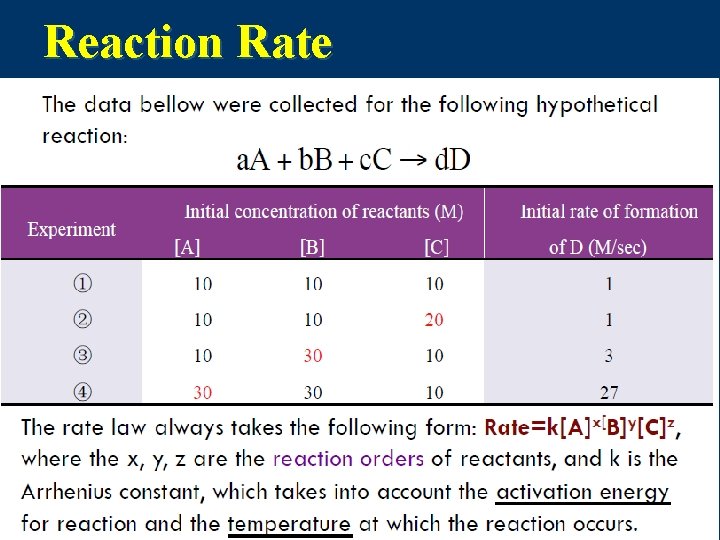
![a. A + b. B+ Cc→d. D n Rate=k[A]2[B] k= Rate/([A]2[B])=(1 M/sec)/[(10 M)2(10 M)] a. A + b. B+ Cc→d. D n Rate=k[A]2[B] k= Rate/([A]2[B])=(1 M/sec)/[(10 M)2(10 M)]](http://slidetodoc.com/presentation_image_h2/274bb6c58ce1d0295173562c6dfffd91/image-7.jpg)
a. A + b. B+ Cc→d. D n Rate=k[A]2[B] k= Rate/([A]2[B])=(1 M/sec)/[(10 M)2(10 M)] =1× 10 -3 M-2 sec-1 n the reaction order of this reaction is 3; the reaction order of reactant A is 2; the reaction order of reactant B is 1; n
![EX rate = k[A][B]2 EX rate = k[A][B]2](http://slidetodoc.com/presentation_image_h2/274bb6c58ce1d0295173562c6dfffd91/image-8.jpg)
EX rate = k[A][B]2
![2. First Order Reaction n Rate=k[A] 斜率 2. First Order Reaction n Rate=k[A] 斜率](http://slidetodoc.com/presentation_image_h2/274bb6c58ce1d0295173562c6dfffd91/image-9.jpg)
2. First Order Reaction n Rate=k[A] 斜率
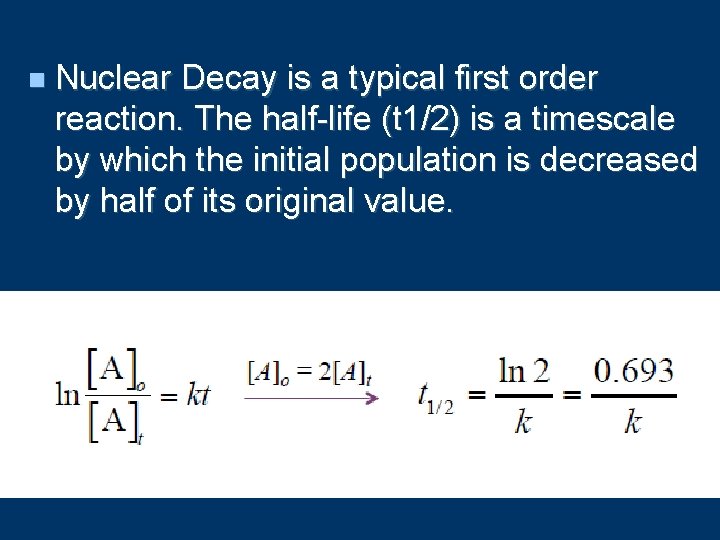
n Nuclear Decay is a typical first order reaction. The half-life (t 1/2) is a timescale by which the initial population is decreased by half of its original value.
![3. Second Order Reaction n Rate=k[A]2 4. Zero Order Reaction n Rate=k 3. Second Order Reaction n Rate=k[A]2 4. Zero Order Reaction n Rate=k](http://slidetodoc.com/presentation_image_h2/274bb6c58ce1d0295173562c6dfffd91/image-11.jpg)
3. Second Order Reaction n Rate=k[A]2 4. Zero Order Reaction n Rate=k

5. Collision Theory 碰撞理论 n Reaction rate depends on the collisions between reacting particles. n Successful collisions occur if the particles. . . · collide with each other · have the correct orientation · have enough kinetic energy to break bonds
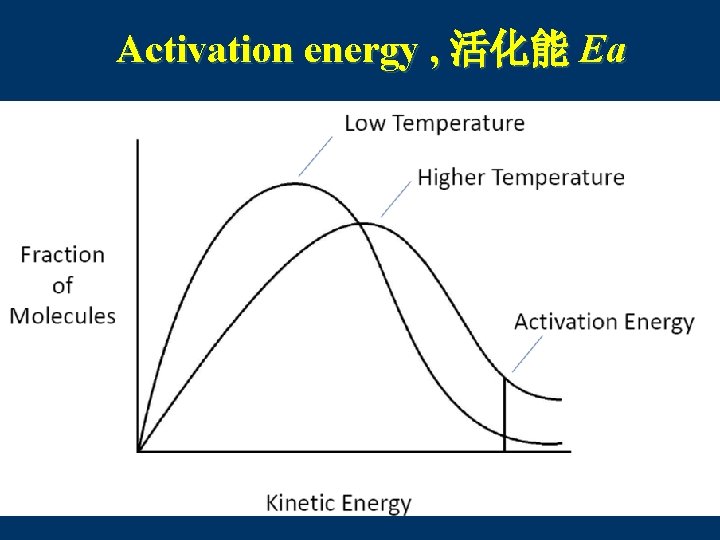
Activation energy , 活化能 Ea C. Johannesson
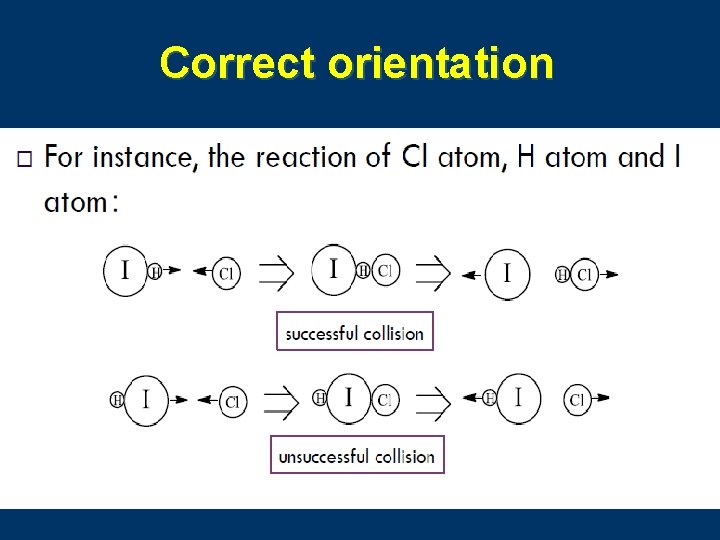
Correct orientation
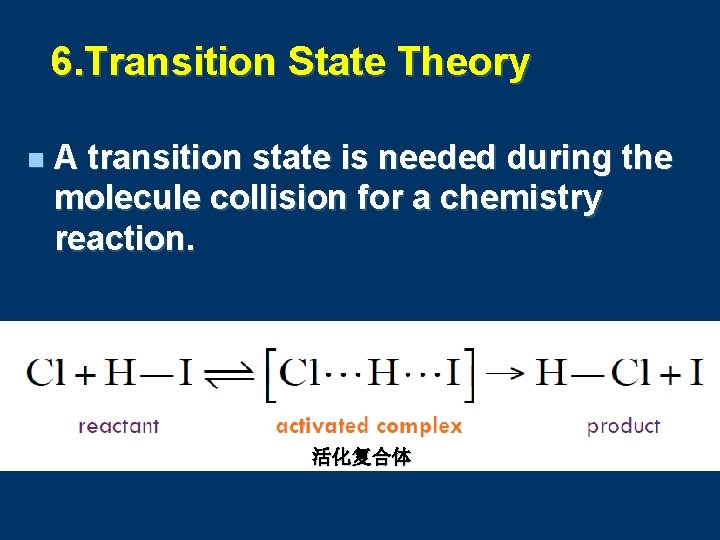
6. Transition State Theory n A transition state is needed during the molecule collision for a chemistry reaction. 活化复合体
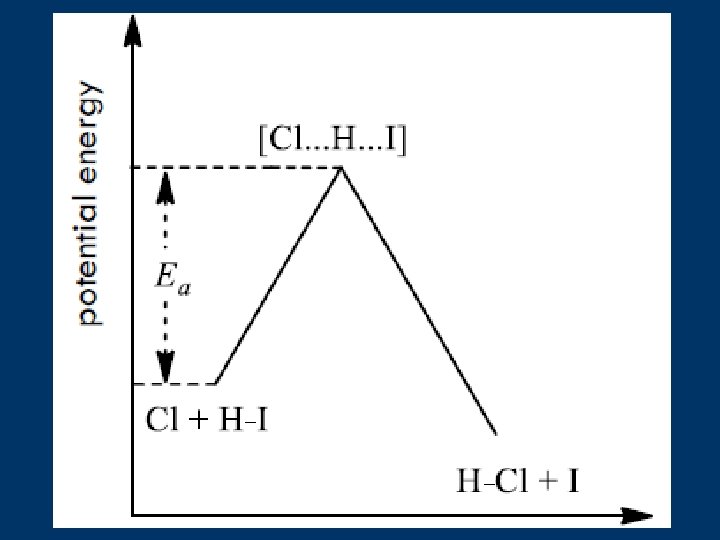
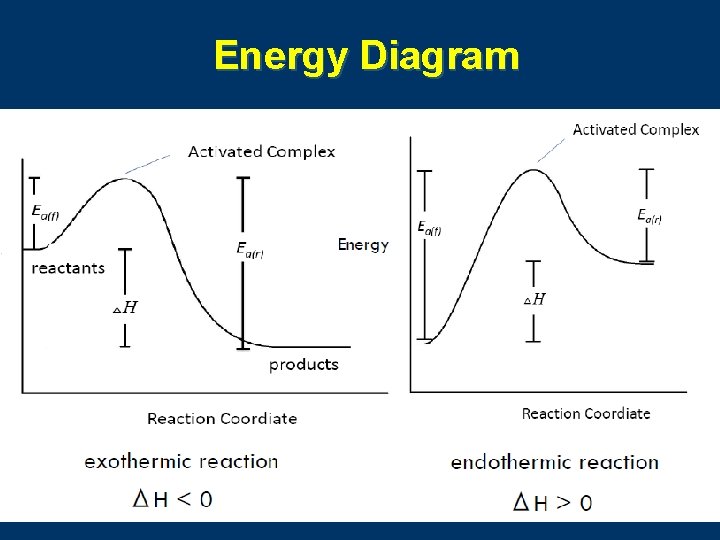
Energy Diagram

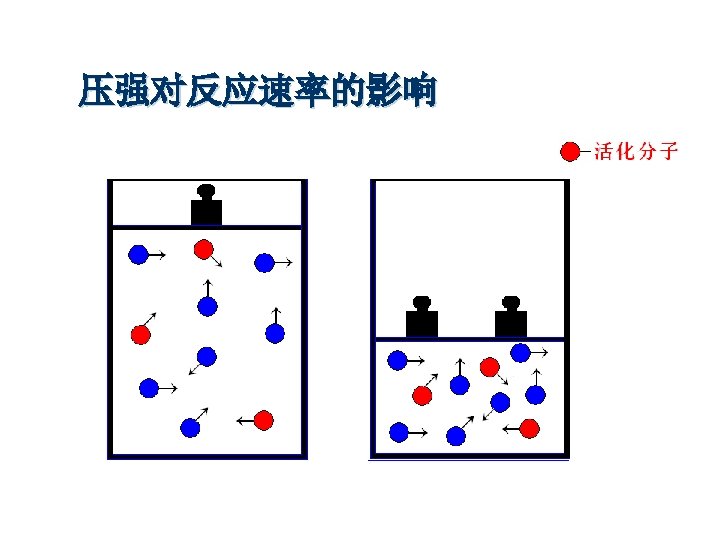
You can make reactions go faster by… “Make more collisions” Temperature Increase Concentration Increase Particle Size Decrease Pressure Increase (Only gases) Increase Catalysts
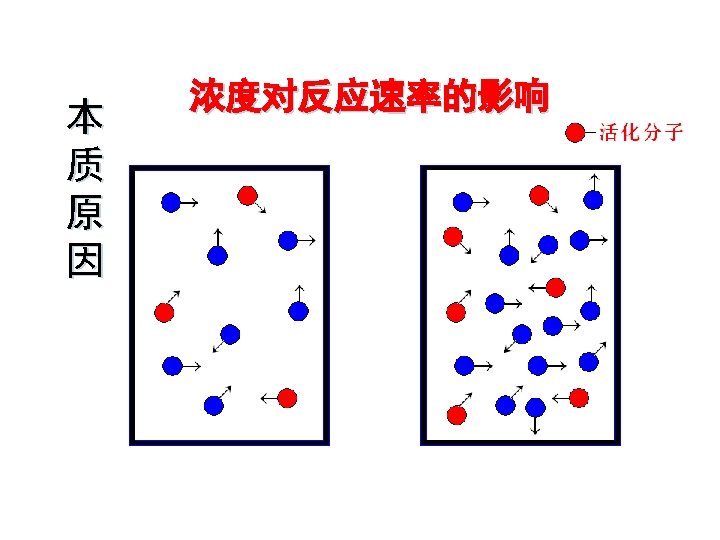

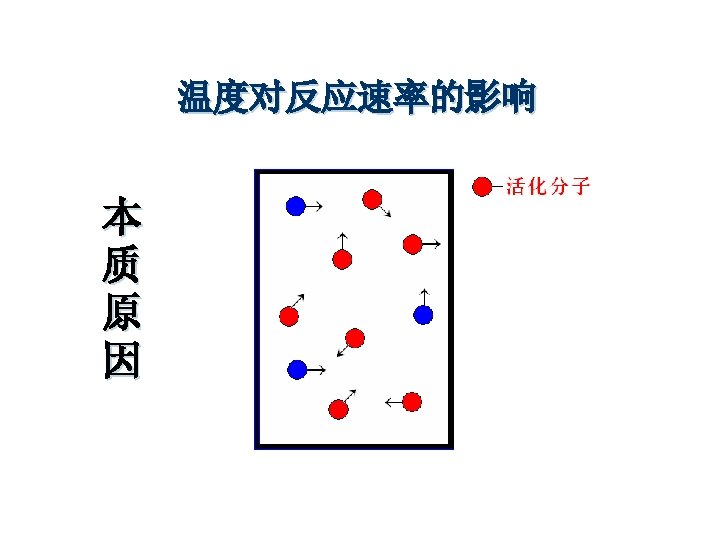
Temperature · high temp = fast rxn rate · high KE - fast-moving particles - more likely to reach activation energy

Surface Area · high SA = fast rxn rate · more opportunities for collisions · Increase surface area by… - using smaller particles - dissolving in water
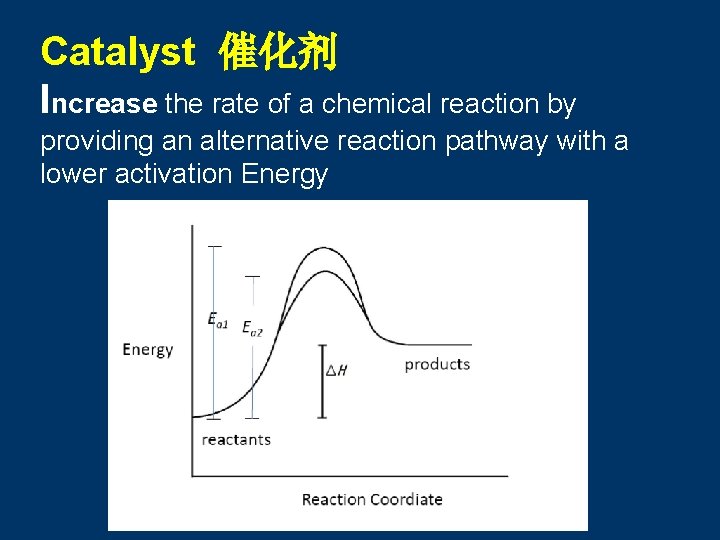
Catalyst 催化剂 Increase the rate of a chemical reaction by providing an alternative reaction pathway with a lower activation Energy
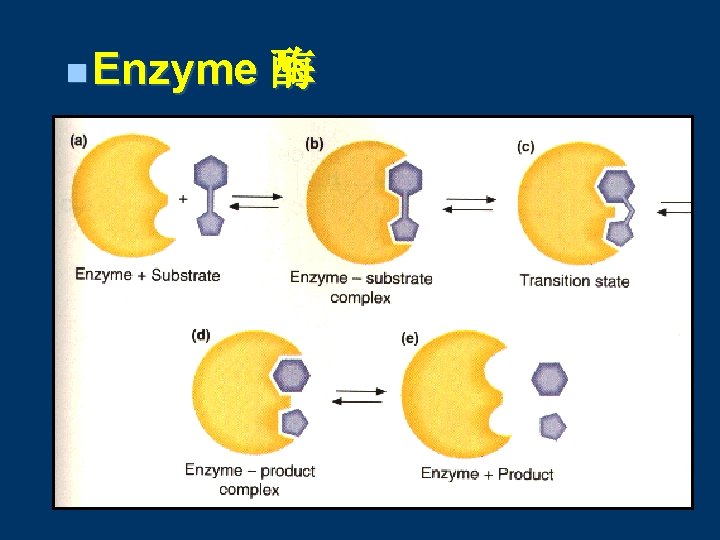
n Enzyme 酶

II. Equilibrium 平衡

Types of equilibrium n Physical l Equilibrium: Phase equilibrium – occurs during a phase change (s) ↔ (l) RATE of Melting = Rate of Freezing (l) ↔ (g) RATE of Evaporation = Rate of Condensation l Solution equilibrium – at a solution’s saturation point RATE of dissolving = Rate of crystallization Example Na. Cl(s) ↔ Na. Cl(aq)

Chemical Equilibrium

1. Reversible Reaction 可逆反应
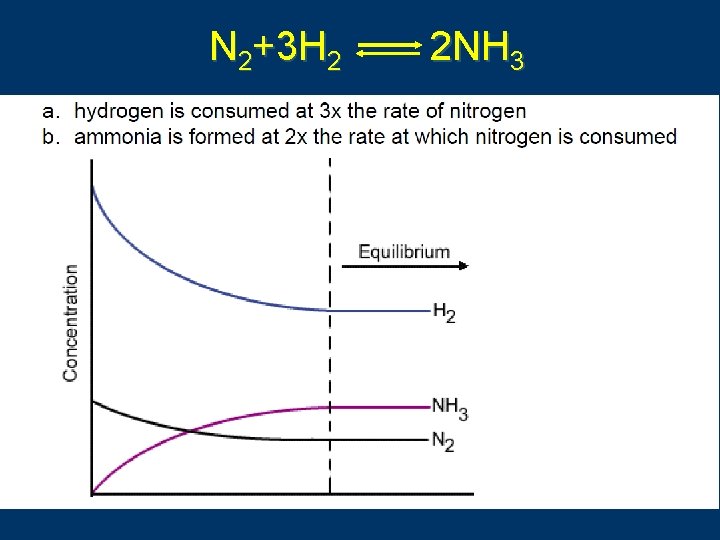
N 2+3 H 2 2 NH 3 C. Johannesson

A reaction is at equilibrium, the concentrations of reactants and products do not change any more. 浓度不再变 In fact, forward reaction and reverse reaction are still in progress with equal reaction rates. 没有停止 Is a dynamic equilibrium. 动态平衡
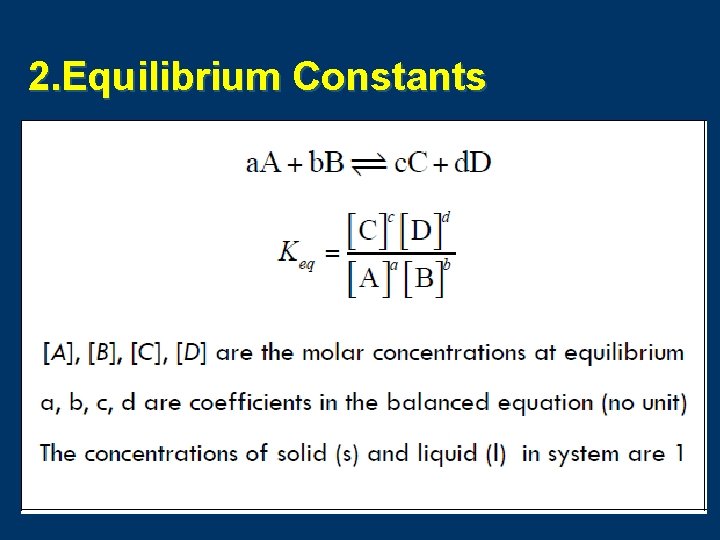
2. Equilibrium Constants
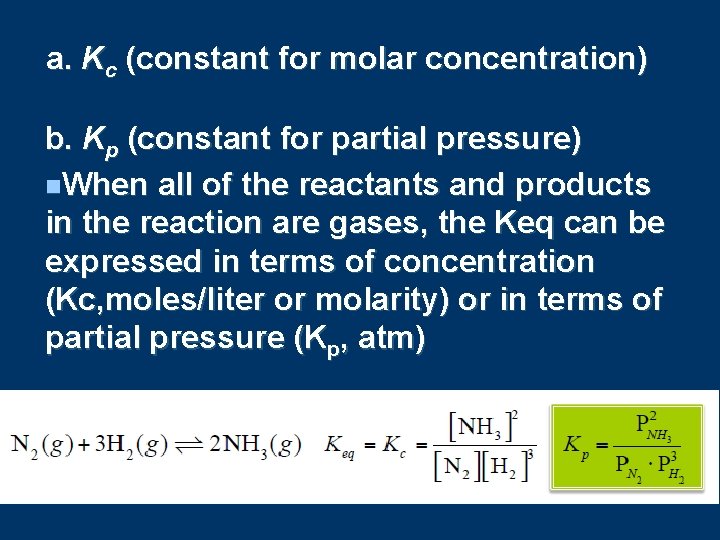
a. Kc (constant for molar concentration) b. Kp (constant for partial pressure) n. When all of the reactants and products in the reaction are gases, the Keq can be expressed in terms of concentration (Kc, moles/liter or molarity) or in terms of partial pressure (Kp, atm)
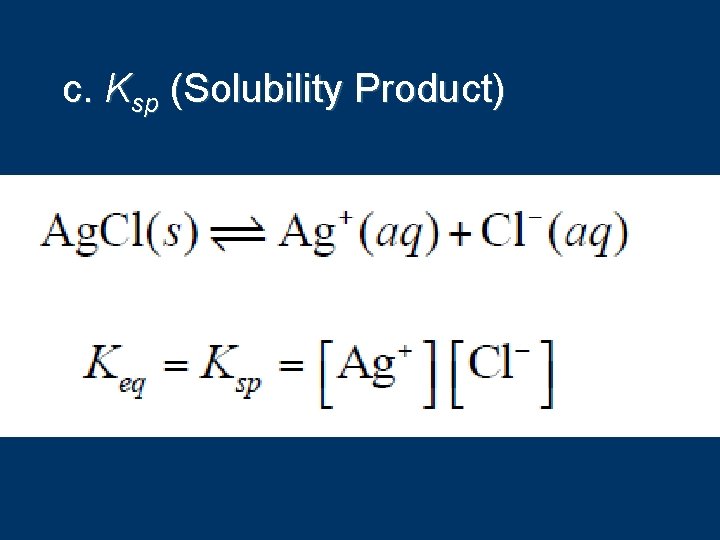
c. Ksp (Solubility Product)
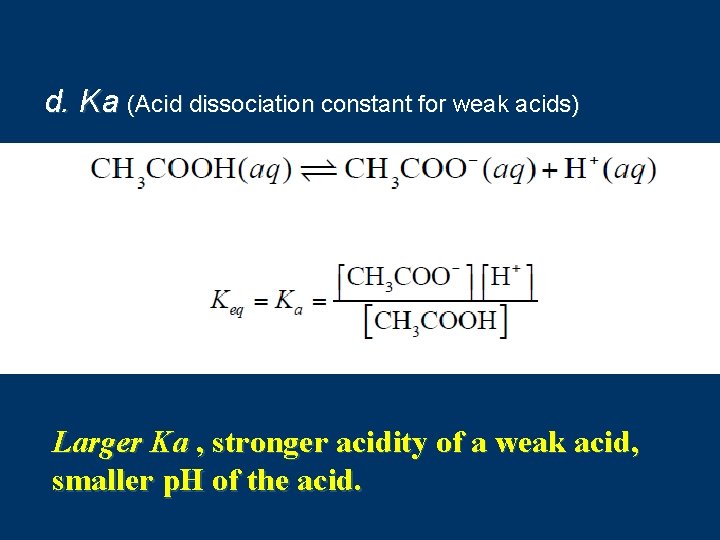
d. Ka (Acid dissociation constant for weak acids) Larger Ka , stronger acidity of a weak acid, smaller p. H of the acid.
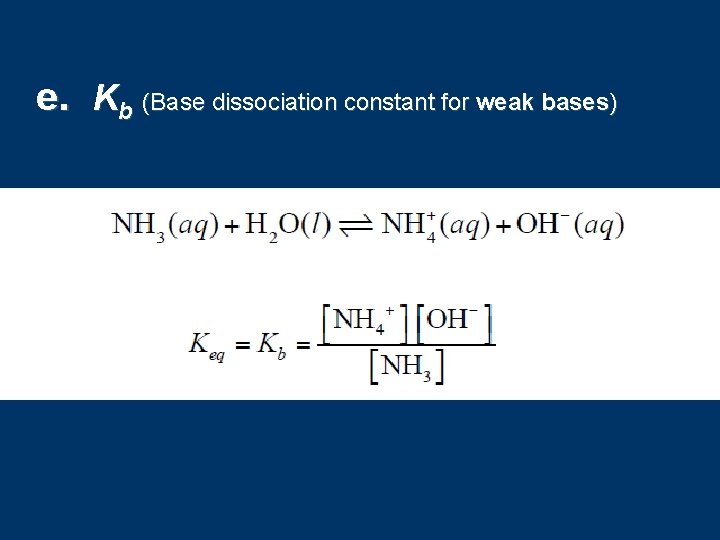
e. Kb (Base dissociation constant for weak bases)
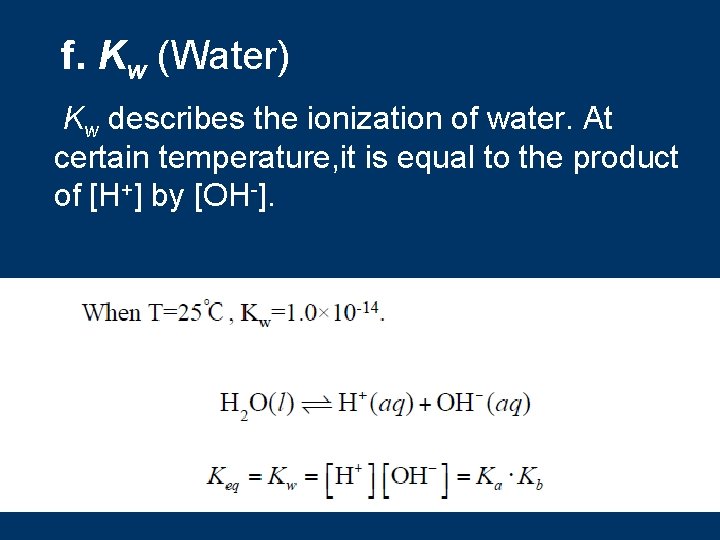
f. Kw (Water) Kw describes the ionization of water. At certain temperature, it is equal to the product of [H+] by [OH-].
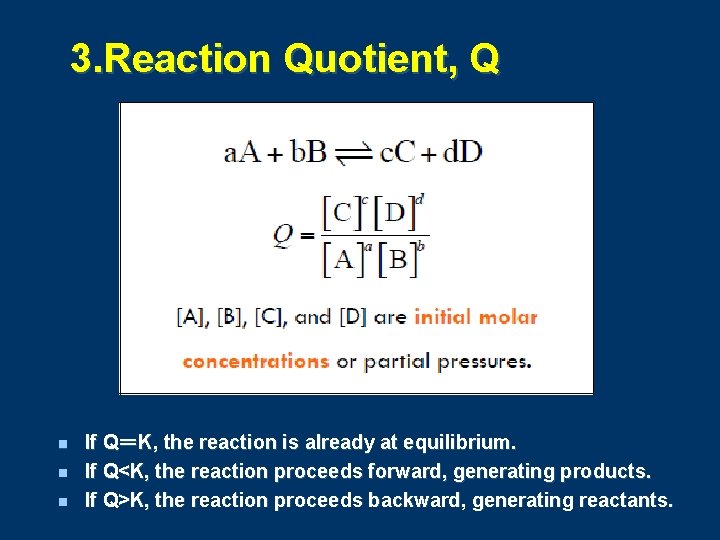
3. Reaction Quotient, Q n n n If Q=K, the reaction is already at equilibrium. If Q<K, the reaction proceeds forward, generating products. If Q>K, the reaction proceeds backward, generating reactants.

4. Le. Châtelier’s Principle Equilibrium Position n At this point the concentrations don’t change unless a stress is applied to change the equilibrium. Le. Châtelier’s Principle When a system at equilibrium is placed under stress, the system will undergo a change in such a way as to relieve that stress.

System stresses: · Concentration of reactants or products · Temperature · Pressure · Catalyst Q? K

a. Concentration When the concentration of a reactant is increased or that of a product is decreased, Q<K, the reaction will proceed forward. n When the concentration of a reactant is decreased or that of a product is increased, Q>K, the reaction will proceed backward. n

b. Pressure (gas) Increased pressure, the reaction proceeds in the direction that produce less moles of gas. Decreased pressure, the reaction proceeds in the direction that produce more moles of gas. n If the reactants and products have the same number of moles of gas, then the pressure changes have no effect on the equilibrium. (moles/volume) n

c. Temperature When temperature is increased, the reaction will proceed in the endothermic direction. n When temperature is decreased, the reaction will proceed in the exothermic direction. n

Van’t Hoff 等温式 ΔG= ΔG 0 + 2. 30 RT lg. Q ΔG=0 -ΔG 0 = 2. 30 RT lg. Q -ΔG 0 /2. 30 RT= lg. Q lg. K= - ΔG 0 /2. 30 RT

EX A closed container of ice and water is at equilibrium. Then, the temperature is raised. Ice + Energy Water The system temporarily shifts to the right _______ to restore equilibrium.
- Slides: 45