CH 10 INTERMOLECULAR FORCES types influences strength LIQUIDS






















- Slides: 41

CH. 10 INTERMOLECULAR FORCES >types >influences >strength LIQUIDS - SOLIDS >physical properties TRENDS PHASES - PHASE CHANGE >heating curves > H calculations Equations Clausius-Claperyron

RECALL 3 physical states solid -- liquid -- gas condensed phases Phase change related to: intermolecular forces + KE PE depends on (Coulomb’s) *charges of particles; dist bet **KEspeed a absol. T Chemical behavior in diff phases same Physical behavior in diff phases diff WHY? ? ? Due to strength of inter- forces

FORCES Intra-F: w/i the molecule Inter-F: bet molecules POINTS most all liquids @ room temp are molecules Intra-F give rise to covalent bonding influence 1) molecular shape 2) bond E’s 3) chem behavior Ch. 8 attraction of gases deviates Ideal Gas Law

PHYSICAL PROPERTIES OF LIQUIDS - SOLIDS Due to inter-F Characteristic Properties of G - L - S understood in terms of …. 1) E of motion (KE) of 2) particles (atoms, molecules, ions) of 3) g-l-s states compared to E of inter-F bet particle GAS E of attraction bet particles <<< KEave >allows gas to expand

LIQUIDS >Inter-F liquid > in gases >hold particles together >denser, less compressible than gases >partilces move among others, allows “pouring” SOLIDS >Inter-F >>> gases/liquids >“lock”particles in rigid form* E & motion >little free space >Crystalline: orderly structured arrangement

Free Space: gas > liquid > solid condensed phase Then how can you state? heating or cooling; KE show the molecules are arranged among themselves in diff phases

EX. Na. Cl @ 1 atm (incr temp) room: solid 801 o. C: melts, liq 1413 o. C: boils, gas N 2 O (decr temp) room: gas -88. 5 o. C: melts, liq -90. 8 o. C: solid

INTERMOLECULAR FORCES Bonding Ionic --- Covalent --- Metallic Forces vary for diff subst Inter-F < Ionic < Covalent E to vaporize liq E to evaporate liq E to melt solid < E break covalent bond

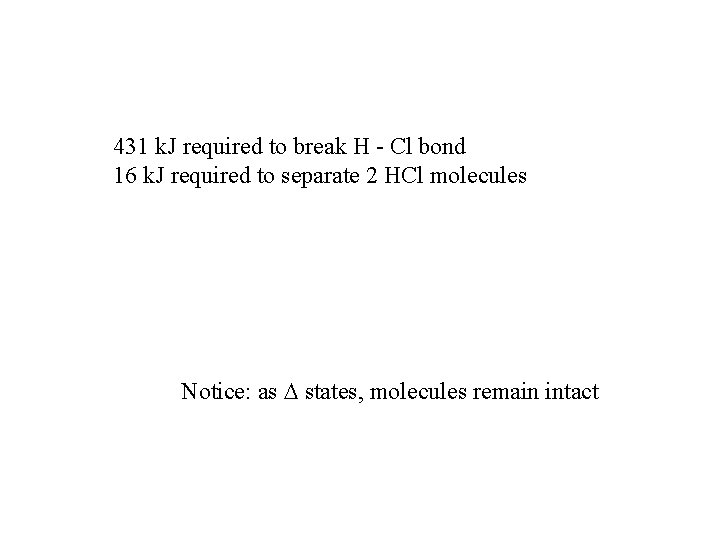
431 k. J required to break H - Cl bond 16 k. J required to separate 2 HCl molecules Notice: as states, molecules remain intact

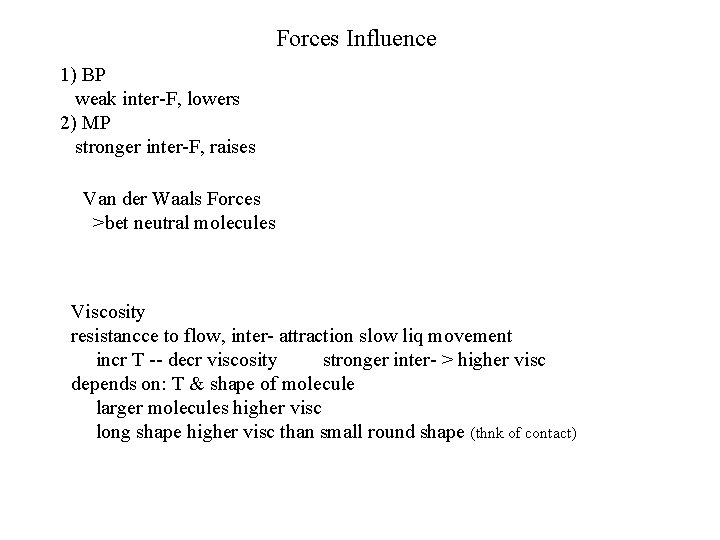
Forces Influence 1) BP weak inter-F, lowers 2) MP stronger inter-F, raises Van der Waals Forces >bet neutral molecules Viscosity resistancce to flow, inter- attraction slow liq movement incr T -- decr viscosity stronger inter- > higher visc depends on: T & shape of molecule larger molecules higher visc long shape higher visc than small round shape (thnk of contact)

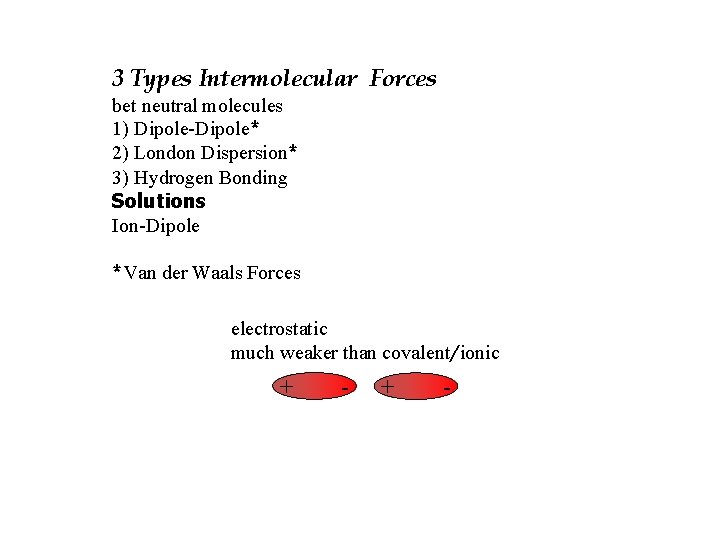
3 Types Intermolecular Forces bet neutral molecules 1) Dipole-Dipole* 2) London Dispersion* 3) Hydrogen Bonding Solutions Ion-Dipole *Van der Waals Forces electrostatic much weaker than covalent/ionic + -

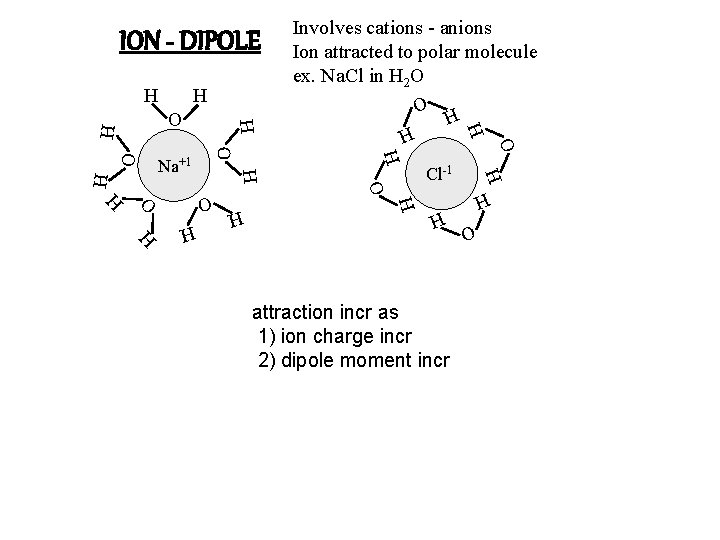
ION - DIPOLE Involves cations - anions Ion attracted to polar molecule ex. Na. Cl in H 2 O H H O O H H H attraction incr as 1) ion charge incr 2) dipole moment incr O H H H O H Cl-1 H O O H H Na+1 H H

similar to ion, but bet neutral charge polar molecules; +/- ends of polar molecule attract DIPOLE - DIPOLE + - + - + D-D < I-D

diff molecules of approx = size & mass: attraction incr w/ incr polarity, BP incr Polar vs Non higher bp More E to overcome forces -- higher bp show fig 11. 7 pg 448

fig. 11. 8 pg. 449 Which subst has the strongest dipole-dipole attraction?

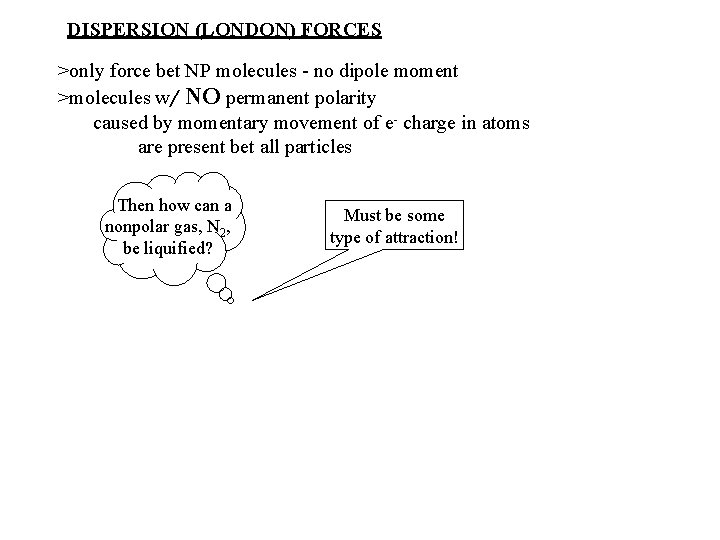
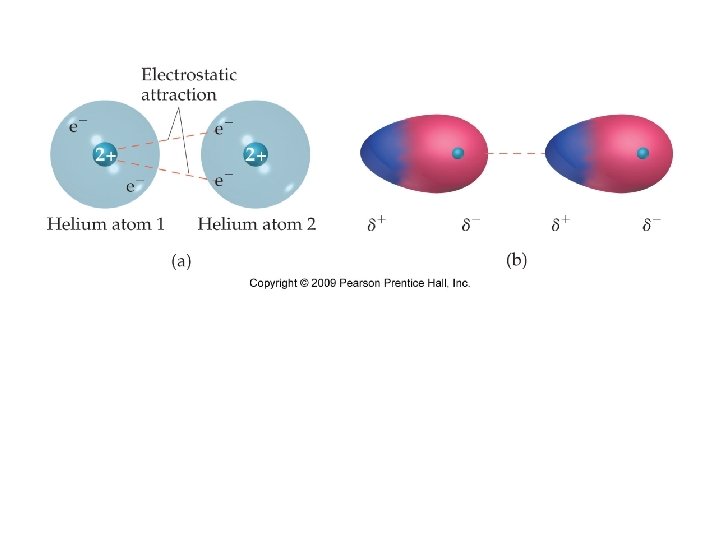
DISPERSION (LONDON) FORCES >only force bet NP molecules - no dipole moment >molecules w/ NO permanent polarity caused by momentary movement of e- charge in atoms are present bet all particles Then how can a nonpolar gas, N 2, be liquified? Must be some type of attraction!

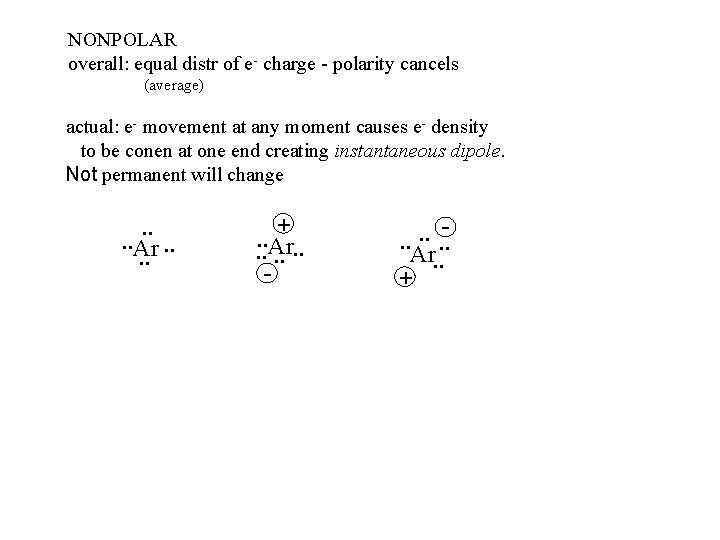
NONPOLAR overall: equal distr of e- charge - polarity cancels (average) actual: e- movement at any moment causes e- density to be conen at one end creating instantaneous dipole. Not permanent will change . . Ar. . +. . Ar. . . - . . . Ar. . +


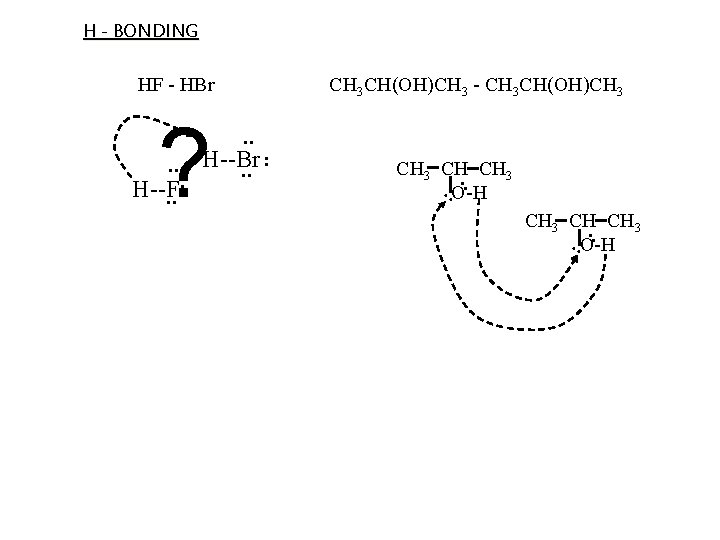
H - BONDING special Dip-Dip when H bonded to small size, high EN atom w/ unbonded e- pair plays imprt role in biological sys Criteria molecule 1: H bonded to O, N, or F molecule 2: unbonded e- on O, N, or F result: H on molecule 1 interact w/ unbonded e- on molecule 2 O . . O H H . . H N H H . . Cl. . F C H . . . . NH 3 - CH 2 FCl . . H 2 O - H 2 O H H

H - BONDING CH 3 CH CH 3. . O-H . . . H--Br . . ? H--F CH 3 CH(OH)CH 3 - CH 3 CH(OH)CH 3 . . HF - HBr

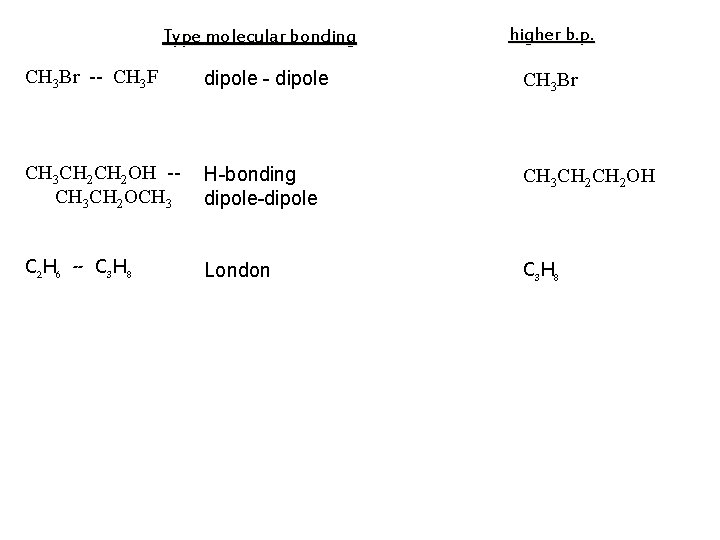
Type molecular bonding higher b. p. CH 3 Br -- CH 3 F dipole - dipole CH 3 Br CH 3 CH 2 OH -CH 3 CH 2 OCH 3 H-bonding dipole-dipole CH 3 CH 2 OH C 2 H 6 -- C 3 H 8 London C 3 H 8

Which has a lower boiling pt in each pair NH 3 -- PH 3, dipole-dipole forces weaker, as stronger H-bonding w/ NH 3 Na. Br -- PBr 3, dipole-dipole forces, as Na. Br stronger ionic bonding H 2 O -- HBr dipole-dipole forces weaker than H-bonding in water

PROPERTIES OF LIQUIDS Surface Tension @ surface molecules attracted only downward (no molecules above), so need KKE to break thru surface stronger forces > surface tension Capillarity liq rises in small space against pull of gravity; forces acting bet cohesive (w/i liq) & adhesive forces Viscosity resistancce to flow, inter- attraction slow liq movement incr T -- decr viscosity stronger inter- > higher visc depends on: T & shape of molecule larger molecules higher visc long shape higher visc than small round shape (thnk of contact)

GAS - no attraction (far apart); random; highly compress; flow/diffuse ezly LIQUID - some attraction (contact); random; not compress; flow/diffuse slower Phase Changes SOLID - strongest attraction (fixed position); not compress; flow/diffuse not


(endo) vaporization gas -------> liq <------- liq ----> solid (endo; fusion) melting <------- condensation (exo) freezing (exo)

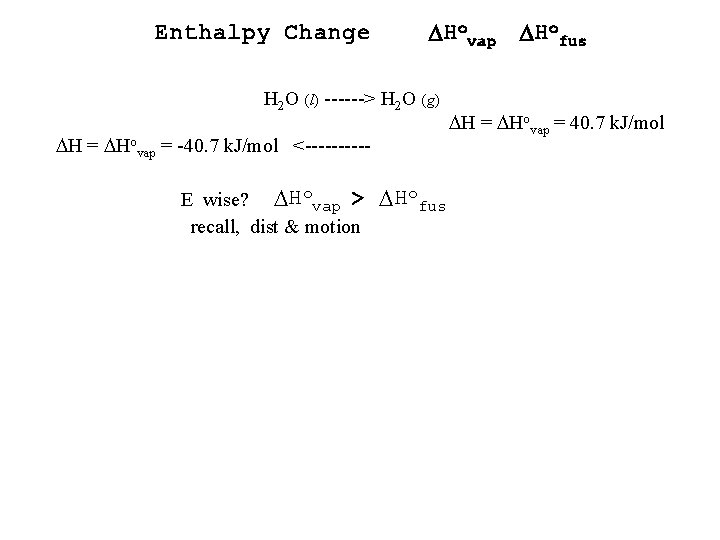
Enthalpy Change DHovap DHofus H 2 O (l) ------> H 2 O (g) H = Hovap = -40. 7 k. J/mol <-----E wise? Hovap > recall, dist & motion Hofus H = Hovap = 40. 7 k. J/mol


VP - depends on T, inter- forces effects of incr T: incr n to vaporize, decr amt condense higher T -- higher vp SOLID - strongest attraction (fixed position); not compress; flow/diffuse not Clausius - Clapeyron Eqn Universal Gas Const R = 8. 31 J/mol-K Hold 3 variables const, vary 1, can find 4 th

At 34. 10 C, vp. H 2 O = 40. 1 torr. Find the vp @ 88. 50 C. Hvap = 40. 7*103 N-m 1 N-m = 1 J P 1 = 40. 1 torr T 1 = 273. 15 + 34. 1 = 307. 25 K T 2 = 361. 65 K P 2 = 11. 0835*(40. 1 torr) = 444 torr Talked about bp - What exactly is bp? -- is the T when ext. P = vp

From the data: bp = 78. 5 o. C cgas = 1. 43 J/g-o. C Hvap = 40. 5 k. J/mol cliq = 2. 45 J/g-o. C At constant P (1 atm), how much heat needed to convert 0. 333 mol of ethanol gas at 300 o. C to liquidfy at 25. 0 o. C A liquid has a VP of 641 torr at 85. 2 o. C, and bp of 95. 6 o. C at 1 atm. Calculate Hvap.

3 steps: gas; gas-liq; liq CH 3 CH 2 OH: 46. 0 g/mol 1 st: find mass: 0. 333 mol*(46. 0 g/mol) = 15. 3 g Cooling vapor to bp: q = Cgas*mass* T = (1. 43 J/g-o. C)*(15. 3 g)*(78. 5 - 300) = -4846 J Condensation (*direction) q = n*(- Hcond) = (0. 333 mol)*(-40. 5 k. J/mol) = -13. 4865 k. J*1000 = -13487 J Cooling liquid to 25. 0 o. C q = Cliq*mass* T = (2. 45 J/g-o. C)*(15. 3 g)*(25. 0 - 78. 5) = -2055 J Total q = qvapor+qcond+qliq = (-4846 J)+(-13487 J)+(-2005 J) = -20, 338 J (-2. 03*104 J)

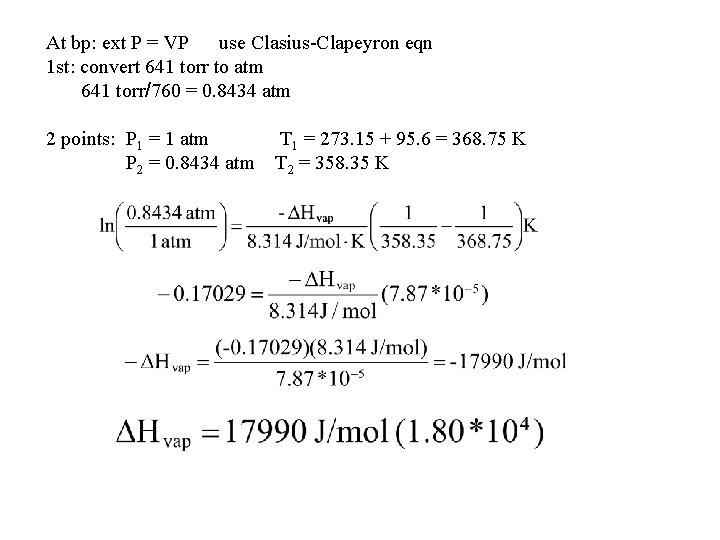
At bp: ext P = VP use Clasius-Clapeyron eqn 1 st: convert 641 torr to atm 641 torr/760 = 0. 8434 atm 2 points: P 1 = 1 atm P 2 = 0. 8434 atm T 1 = 273. 15 + 95. 6 = 368. 75 K T 2 = 358. 35 K

COOLING CURVE Shows changes that occur when add/remove heat @ const T fig 11. 22, pg 440

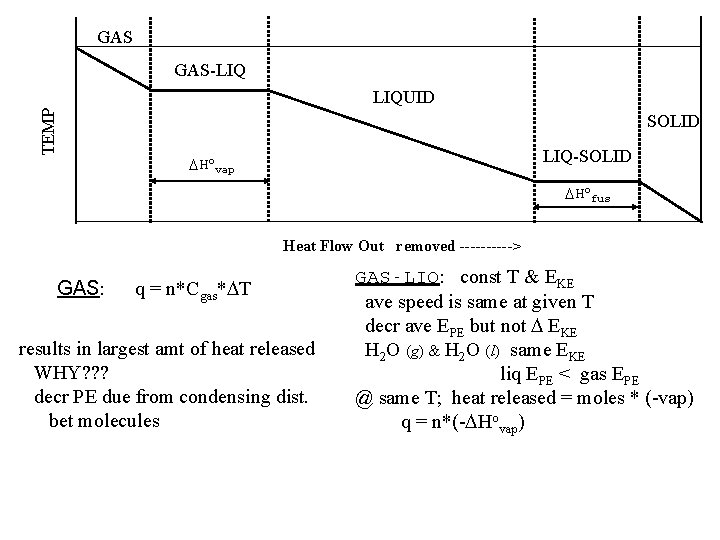
GAS GAS-LIQ TEMP LIQUID SOLID LIQ-SOLID Hovap Hofus Heat Flow Out removed -----> GAS: q = n*Cgas* T results in largest amt of heat released WHY? ? ? decr PE due from condensing dist. bet molecules GAS-LIQ: const T & EKE ave speed is same at given T decr ave EPE but not EKE H 2 O (g) & H 2 O (l) same EKE liq EPE < gas EPE @ same T; heat released = moles * (-vap) q = n*(- Hovap)

LIQUID q = n*Cliq* T * loss of heat results in decr T decr molecular speed, this decr EKE LIQ-SOLID * inter- attraction > motion of molecules * loss EPE form crystalline solid * const T & EKE * H 2 O (l) & H 2 O (soln) same EKE solid EPE < liq EPE @ same T; heat released = moles * (-fusion) q = n*(- Hofus) SOLID q = n*Csol* T motion restricted; decr T reduced ave speed TOTAL HEAT RELEASED Use Hess’ Law sum of 5 steps 2 pts @ const P w/i phase: q is T ( EKE) depends on: amt subst (n), C for phase, T during phase : q (@T)( EPE), dist bet molecules changes

LIQ-GAS EQUILIBRA @ const T open closed Sys reaches pt of dynamic balance @ equilibria & vp const vaporize condense Weaker bonds = higher vp, lower bp Keep in Mind: when a sys at equil is distr, will react in way to counteract said disturb to regain a state at a new equilib

SOLID - GAS EQUILIBRA: Sublimation Solids decr vp < liq Solids: high vp Phase Diagrams? ? ?

SUMMARY CH. 11 Clausius-Claperyron EQUILIBRA q = n*CPHASE* T gas -------> liq condensation (exo) (endo) vaporization <------liq ----> solid (endo; fusion) melting <------- COOLING CURVE INTERMOLECULAR FORCES bond type; bp/mp higher/lower freezing (exo)

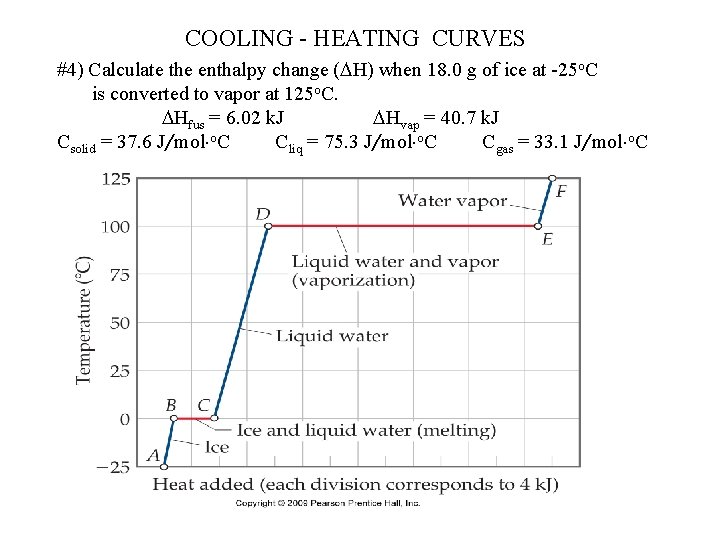
COOLING - HEATING CURVES #4) Calculate the enthalpy change ( H) when 18. 0 g of ice at -25 o. C is converted to vapor at 125 o. C. Hfus = 6. 02 k. J Hvap = 40. 7 k. J Csolid = 37. 6 J/mol o. C Cliq = 75. 3 J/mol o. C Cgas = 33. 1 J/mol o. C

4) 5 steps: solid; solid-liq; liquid; liq-gas; gas given 18. 0 g = 1 mol *** look at labels on “C” Heat solid to mp: q = Csolid*mol* T = (37. 6 J/mol-o. C)*(1. 00 mol)*(0 - -25) = 940 J Fusion (*direction) q = n*( Hfus) = (1. 00 mol)*(6. 02 k. J/mol) = 6. 02 k. J*1000 = 6020 J Heating liquid to bp q = Cliq*mol* T = (75. 2 J/mol-o. C)*(1. 00 mol)*(100. 0 - 0. 0) = 7520 J Vaporization (*direction) q = n*( Hvap) = (1. 00 mol)*(40. 7 k. J/mol) = 40. 7 k. J*1000 = 40700 J Heat gas to 125 o. C q = Cgas*mol* T = (33. 1 J/mol-o. C)*(1. 00 mol)*(125. 0 - 100. 0) = 830 J Total q = qsolid + qfus + qliq + qvap + qgas = (940 J)+(6020 J)+(7520 J)+(40700 J)+(830 J) = 56, 000 J (56 k. J)