Ch 10 Chemical Quantities 10 1 The Mole







- Slides: 14

Ch. 10: Chemical Quantities

10. 1: The Mole I. Mole (mol): a measurement of matter; equal to 6. 02 x 1023 representative particles of a substance, which is Avogadro’s Number; the SI unit for measuring the amount of a substance. A. A mole of any substance contains Avogadro’s number of representative particles, or 6. 02 x 1023 representative particles. B. Conversions: Particles moles; moles particles

C. The Mass of a Mole of an Element: varies with atomic mass, but the number of particles remains the same; units: amu = g/mol. 1. Carbon: 12. 0 amu and/or 12. 0 g has 6. 02 x 1023 atoms and equals 1 mol. 2. Hydrogen: 1. 0 amu and/or 1. 0 g has 6. 02 x 1023 atoms and equals 1 mol. 3. Chlorine: 35. 5 amu and/or 35. 5 g has

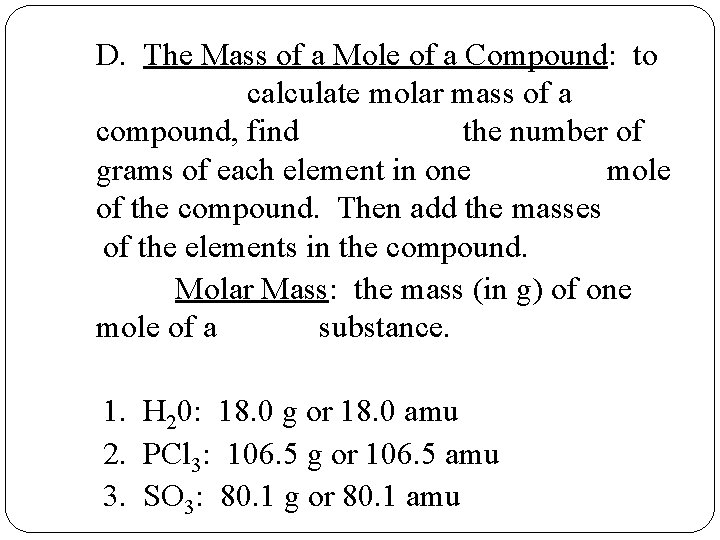
D. The Mass of a Mole of a Compound: to calculate molar mass of a compound, find the number of grams of each element in one mole of the compound. Then add the masses of the elements in the compound. Molar Mass: the mass (in g) of one mole of a substance. 1. H 20: 18. 0 g or 18. 0 amu 2. PCl 3: 106. 5 g or 106. 5 amu 3. SO 3: 80. 1 g or 80. 1 amu

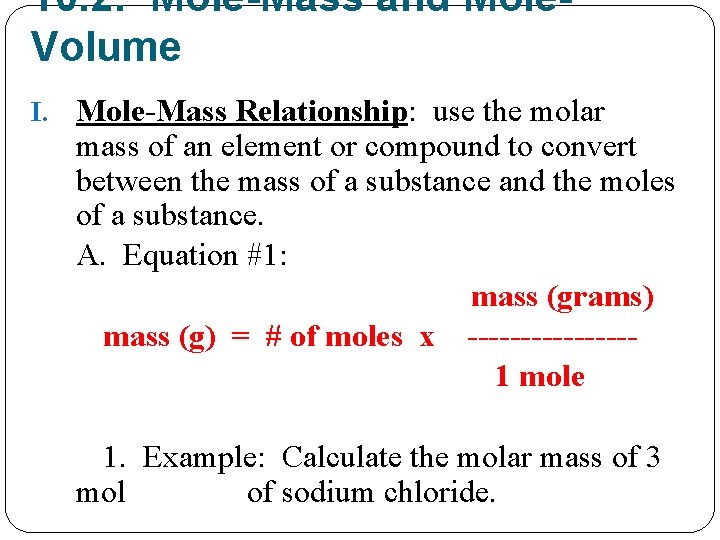
10. 2: Mole-Mass and Mole. Volume I. Mole-Mass Relationship: use the molar mass of an element or compound to convert between the mass of a substance and the moles of a substance. A. Equation #1: mass (grams) mass (g) = # of moles x --------1 mole 1. Example: Calculate the molar mass of 3 mol of sodium chloride.

B. Equation #2: moles = mass (g) x 1 mole --------mass (grams) 1. Example: Calculate the number of moles of iron(III) oxide in 92. 2 g of iron(III) oxide. II. Mole-Volume Relationship: At STP, 1 mol or 6. 02 x 1023 particles, of any gas occupies a volume of 22. 4 L (molar volume).

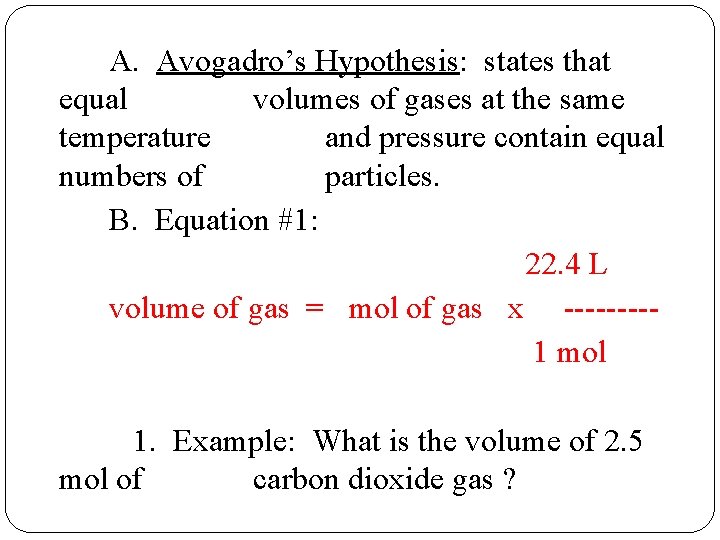
A. Avogadro’s Hypothesis: states that equal volumes of gases at the same temperature and pressure contain equal numbers of particles. B. Equation #1: 22. 4 L volume of gas = mol of gas x ----1 mol 1. Example: What is the volume of 2. 5 mol of carbon dioxide gas ?

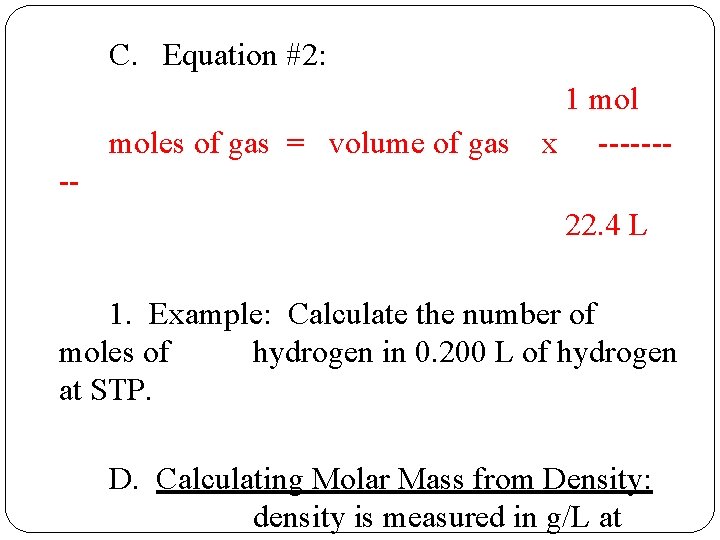
C. Equation #2: moles of gas = volume of gas 1 mol x ------- -22. 4 L 1. Example: Calculate the number of moles of hydrogen in 0. 200 L of hydrogen at STP. D. Calculating Molar Mass from Density: density is measured in g/L at

1. Equation: 22. 4 L Molar mass (g/mol) = density (g/L) x -------1 mole a. Example: What is the molar mass of a compound containing carbon and oxygen that has a density of 1. 964 g/L at STP ?

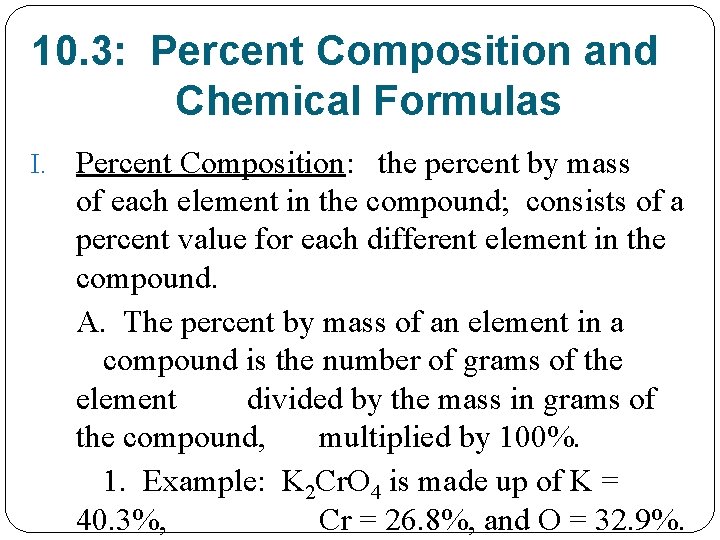
10. 3: Percent Composition and Chemical Formulas I. Percent Composition: the percent by mass of each element in the compound; consists of a percent value for each different element in the compound. A. The percent by mass of an element in a compound is the number of grams of the element divided by the mass in grams of the compound, multiplied by 100%. 1. Example: K 2 Cr. O 4 is made up of K = 40. 3%, Cr = 26. 8%, and O = 32. 9%.

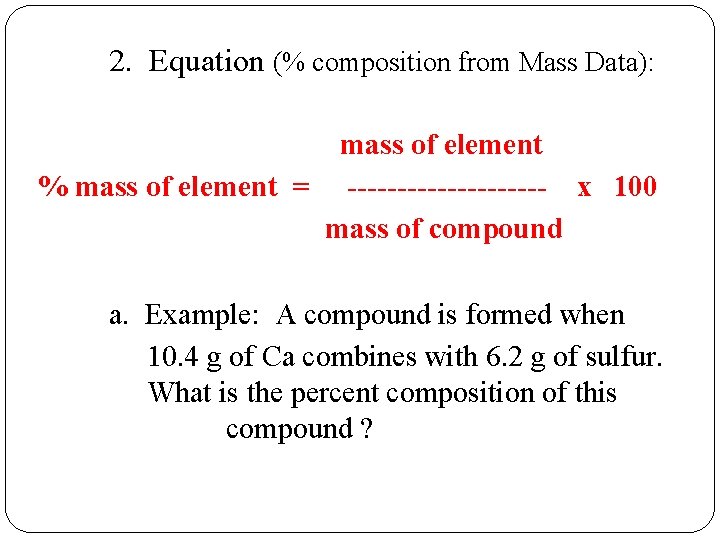
2. Equation (% composition from Mass Data): mass of element % mass of element = ---------- x 100 mass of compound a. Example: A compound is formed when 10. 4 g of Ca combines with 6. 2 g of sulfur. What is the percent composition of this compound ?

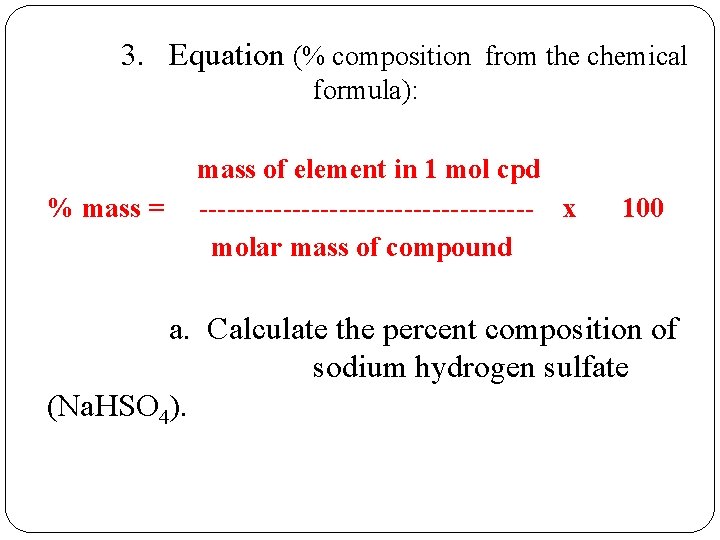
3. Equation (% composition from the chemical formula): % mass = mass of element in 1 mol cpd ------------------ x molar mass of compound 100 a. Calculate the percent composition of sodium hydrogen sulfate (Na. HSO 4).

II. Empirical Formula: the basic molecular ratio of atoms in a compound in relative terms. It indicates the lowest whole number ratio of the atoms of the elements in a compound; the empirical formula may or may not be the same as the molecular formula. A. Examples: 1. Hydrogen Peroxide (H 2 O 2): ratio is 2: 2, but the lowest ratio is 1: 1, therefore the empirical formula is HO. 2. C 2 H 2 and C 8 H 8: lowest ratio is 1: 1, therefore the empirical formula for both is CH.

III. Molecular Formula: formula that is either identical to the empirical formula or is a simple whole-number multiple of its empirical formula; takes molar masses into consideration; indicates exact ratios between atoms within a compound. A. Examples: 1. CH: empirical 2. C 2 H 2: molecular 3. C 2 H 4 O 2: molecular 4. Fe 2(SO 4)3: molecular 5. Mg. O: empirical and molecular