Ch 1 Intro to Chemistry What is Chemistry









- Slides: 26

Ch. 1 Intro to Chemistry

What is Chemistry? • Matter = anything that has mass and occupies space • Chemistry = study of matter and the changes it undergoes • *Chem. Demo*

Areas of Study • 5 Types of Chemistry: 1. Inorganic – study of chemicals that do not contain carbon (nonliving) 2. Organic – study of chemicals containing carbon 3. Biochemistry – study of processes that take place in organisms 4. Analytical – focuses on the composition of matter 5. Physical – area that deals w/the mechanism, the rate, and the energy transfer that occurs when matter undergoes a change (highly theoretical, studies scientific principles

Carbon Dating

Pure vs. Applied Chemistry • Pure chemistry = pursuit of chemical knowledge for its own sake (no immediate application) • Applied chemistry = research that is directed toward a practical goal or application • *Which is more common? • *Break into groups, 1 min. to list careers that would benefit from a knowledge of chemistry*

Careers Health care Chemical engineer Public works Teacher Environmental Turf management Chef, nurse, firefighter, farmer… • Forensics • • Uses • Health and Medicine – Sanitation systems – Surgery w/ anesthesia – Vaccines and antibiotics • Energy and the Env. – Fossil fuels – Solar energy – Nuclear energy • Materials and technology – Polymers, ceramics, liquid crystals – Room-temp superconductors? – Molecular computing? • Food and agriculture – Genetically modified crops – “natural” pesticides – Specialized fertilizers *Write down careers you are considering, describe activities, how would a knowledge of chemistry help

Lab Equipment and Safety

Scientific Method • • 1. Observation 2. Problem/Question 3. Make a hypothesis 4. Test hypothesis/experiment – – Independent variable/manipulated Dependent variable/responding Control group Constants • 5. Analyze data/results • 6. Draw conclusions – Theory – Scientific law/principle • *In-class Bio Example* Old Book SP 1, ASP 1, PP 2 pg. 10 -11

Quick Lab • Using the scientific method pg. 23 – Set up your lab notebooks today and fill them out as if this was a major lab – Check next slide for rules on setting up your lab notebook • Math refresher – Sample problem 1. 1 pg. 30 – Discuss conceptual problem 1. 1 – worksheet

Mr. Kirwan’s Rules for Rearranging Equations • 1) What are you solving for? • 2) Is it on top or bottom? It needs to be on top. If it’s not, move to the other side. • 3) Get it by itself. When letters move sides, they move from top to bottom and vice versa.

Rules for setting up a lab notebook • • The pages are to be consecutively numbered. No pages are ever to be removed All entries are to be made directly in the notebook in black or blue ink. On the first page of your notebook are written the name of the class, your laboratory section and your name. The next two pages are reserved for a table of contents. The words “Table of Contents” are to be written at the top of these pages. The first entry is to be the table of contents itself. An entry is made in the Table of Contents for each experiment when it is begun. This entry includes the title of each experiment and the page number on which the experiment begins. At the top of each page write the title of the experiment that matches that in the table of contents. At the bottom of the page place the date that the last entry was made on that page, your printed name and signature (or initials). If an error is made, draw a single bold horizontal line through the error so that it can still be read. Write the correct information to the right of the incorrect entry and have a short accompanying explanation of the reason for exclusion. Each experiment’s record includes the following sections: Title, Statement of Purpose, Background, Procedural Outline, Results, Calculations, Discussion of Conclusions and Error Analysis, and Summary of Results. Each section should be clearly labeled with the underlined words indicated below. Sign and date each page as it is completed. The Title, Statement of Purpose, Background and Procedural Outline sections must be prepared prior to the laboratory period Next page - - -

• • • Title: This should include the experiment’s title, your name, the name(s) of your lab partner(s), and the date the experiment was begun. Statement of Purpose: Clearly and concisely (two or three complete sentences) describe the purpose of the experiment, including the general method that will be used anticipated results. Background: balanced chem. equations, physical properties of materials, hazardous materials Procedural Outline: This section is a brief , but complete, description of the steps taken to carry out the experiment. Use your own words, You may use a bulleted list for the steps. Results: Tables, graphs Calculations: An example of each calculation performed to reach the final reported answers should be shown with the units clearly shown at each step Discussion of Conclusions and Error Analysis: Summarize your results paralleling what you set forth in the Statement of Purpose, compare them to the expected results. Once you have identified a source of error in your measurement, evaluate how it affected the result, and then suggest how this error could be minimized or eliminated. Summary of Results: For measurement and synthetic exercises you will need to include a final table summarizing the results of your experiment. For a measurement exercise this table should include each individual value used in the establishment of the average (check with your instructor if you have more than three or four individual values), the standard deviation, and the confidence limit. For a synthetic exercise your summary table should include the percent yield and the measured physical properties of the new substance. Once the Summary of Results has been recorded, sign and date the experiment.

Ch. 2 Matter and Change

Properties of Matter • Extensive vs. intensive – extensive depends on the amount, e. g. volume, mass, length – intensive depends on type of matter, e. g. density, temp. , color • Mass = measure of the amount of matter that each object contains • Volume = measure of the space occupied by the object • Physical property = a quality or condition of a substance that can be observed or measured w/o changing the substance’s composition – state, color, hardness, melting pt. , boiling pt. , conductivity, malleability, density, ductility – *what are the properties of printing paper* • Substance = matter that has a definite and uniform composition – Pure substance

States of Matter • • 3 states: solid, liquid, gas Solid – definite shape, definite volume Liquid – indefinite shape, definite volume Gas – indefinite shape, indefinite volume – Gas vs. Vapor - Gas is a substance like oxygen or helium that exists in that state at room temperature, vapor describes the gaseous state of a substance usually a solid or liquid

Physical Change • Physical change = some properties of a material change but the composition of the material does not – Ex: Reversible changes: boil, freeze, melt, condense – Irreversible changes: break, split, grind, cut, crush

Classifying Mixtures • Mixture = a combo of 2 or more substances in which the substances retain their distinct identities • Heterogeneous = composition is not uniform throughout – EX: sugar/sand, salt/pepper, pizza, … – Colloid – particles do not settle out, particle size is small enough to look like a solution but they stay suspended and do not dissolve Ex: blood, fog, milk, mayo, smoke, butter, paint, ink – Suspension – particles settle out and form layers, particle size is larger and mostly are visible Ex: sand/water, oil/water, oj w/ pulp – 2 or more phases – Particles not the same size • Homogeneous (solution) = composition is uniform throughout – – – EX: kool-aid, metal alloy, salt water Solute = substance dissolved, EX: salt in a salt-water solution Solvent = substance in which the solute dissolves, EX: water in salt-water 1 phase Particles the same size

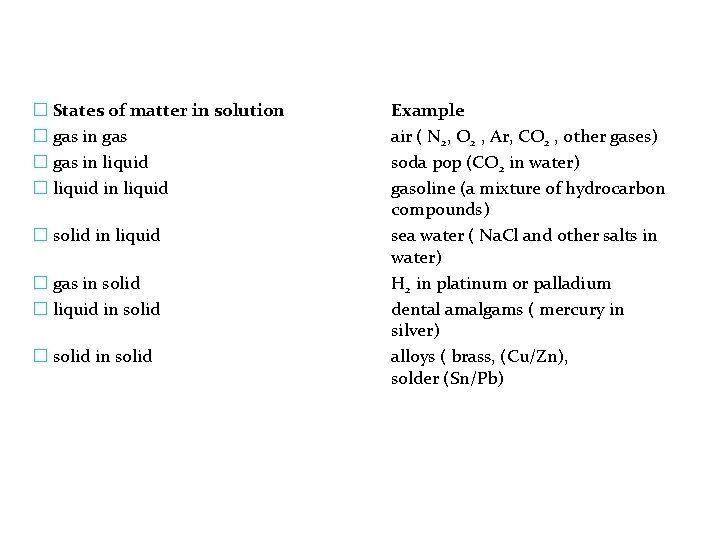
� States of matter in solution � gas in gas � gas in liquid � liquid in liquid � solid in liquid � gas in solid � liquid in solid � solid in solid Example air ( N 2, O 2 , Ar, CO 2 , other gases) soda pop (CO 2 in water) gasoline (a mixture of hydrocarbon compounds) sea water ( Na. Cl and other salts in water) H 2 in platinum or palladium dental amalgams ( mercury in silver) alloys ( brass, (Cu/Zn), solder (Sn/Pb)

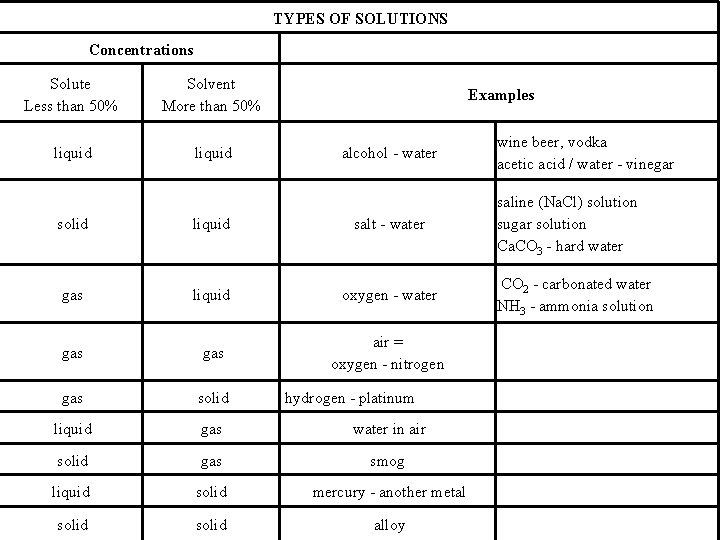
TYPES OF SOLUTIONS Concentrations Solute Less than 50% Solvent More than 50% liquid Examples alcohol - water wine beer, vodka acetic acid / water - vinegar saline (Na. Cl) solution sugar solution Ca. CO 3 - hard water solid liquid salt - water gas liquid oxygen - water gas air = oxygen - nitrogen gas solid liquid gas water in air solid gas smog liquid solid mercury - another metal solid alloy hydrogen - platinum CO 2 - carbonated water NH 3 - ammonia solution

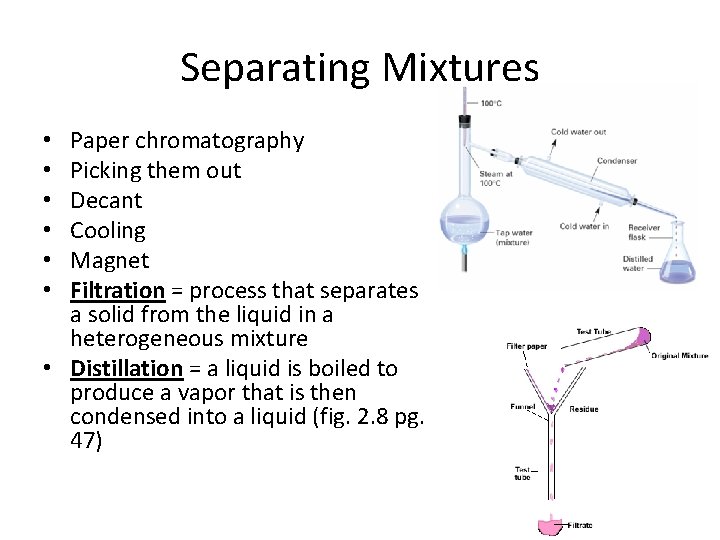
Separating Mixtures Paper chromatography Picking them out Decant Cooling Magnet Filtration = process that separates a solid from the liquid in a heterogeneous mixture • Distillation = a liquid is boiled to produce a vapor that is then condensed into a liquid (fig. 2. 8 pg. 47) • • •

Elements and Compounds • Element = simplest form of matter that has a unique set of properties (pure substance) – cannot be broken down into simpler form – Hydrogen, oxygen, gold, nickel, mercury • Compounds = substance that contains 2 or more elements chemically combined in a fixed proportion – can be broken down by chemical means – Sucrose, water, salt – *Burn Mg strip to form Mg. O – *Name elements, compounds, properties: H 20, Mg. O, Na. Cl, Fe 2 O 3, HCl, Ca. CO 3 (chalk), Na. HCO 3 (baking soda) • Chemical change = change that produces matter w/ a different composition than the original matter – Heating, electricity, oxidation (rust)

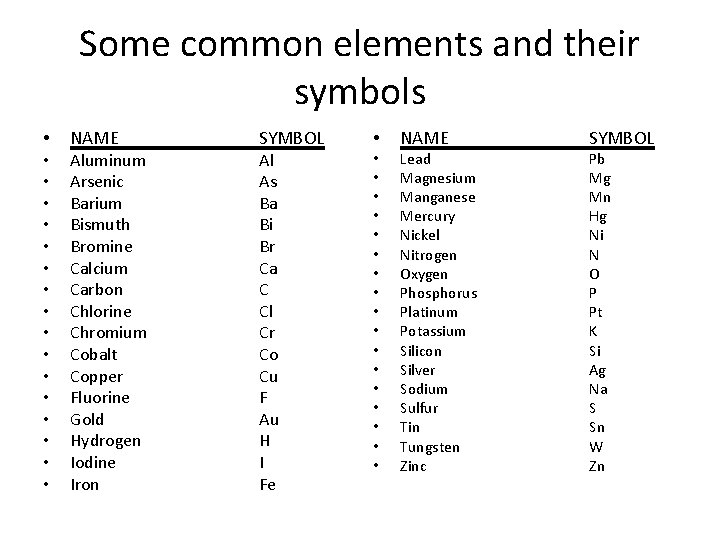
Some common elements and their symbols • • • • • NAME Aluminum Arsenic Barium Bismuth Bromine Calcium Carbon Chlorine Chromium Cobalt Copper Fluorine Gold Hydrogen Iodine Iron SYMBOL Al As Ba Bi Br Ca C Cl Cr Co Cu F Au H I Fe • • • • • NAME Lead Magnesium Manganese Mercury Nickel Nitrogen Oxygen Phosphorus Platinum Potassium Silicon Silver Sodium Sulfur Tin Tungsten Zinc SYMBOL Pb Mg Mn Hg Ni N O P Pt K Si Ag Na S Sn W Zn

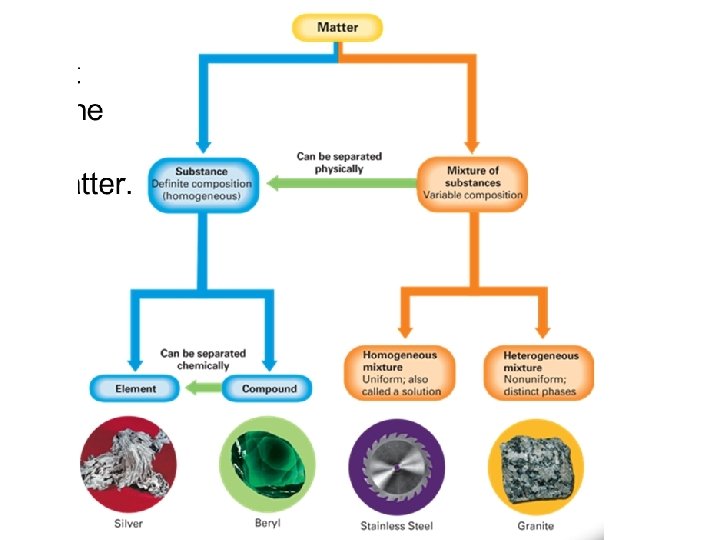
Substances and Mixtures • If the composition of a material is fixed, the material is a substance, if the composition of a material may vary, the material is a mixture • Fig. 2. 11 pg. 50


Chemical Changes • Signifying words for chemical changes: burn, rot, rust, decompose, ferment, explode, corrode • Chemical property = the ability of a substance to undergo a specific chemical change – Ex: ability of iron to rust – Observed only when a substance undergoes a chemical change • Chemical reaction = one or more substances change into one or more new substances – Reactant = substance present at the start (left side) – Product = substance produced in the reaction (right side)

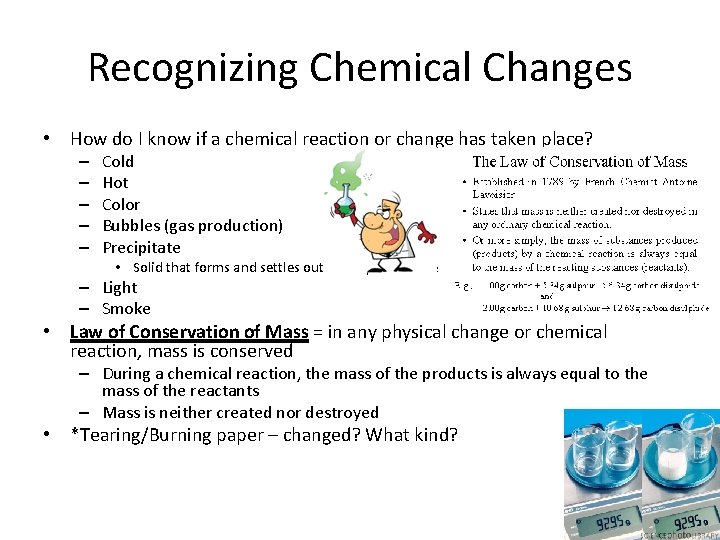
Recognizing Chemical Changes • How do I know if a chemical reaction or change has taken place? – – – Cold Hot Color Bubbles (gas production) Precipitate • Solid that forms and settles out of a liquid mixture – Light – Smoke • Law of Conservation of Mass = in any physical change or chemical reaction, mass is conserved – During a chemical reaction, the mass of the products is always equal to the mass of the reactants – Mass is neither created nor destroyed • *Tearing/Burning paper – changed? What kind?