CH 1 Atomic and Molecular Structure The Periodic






- Slides: 23

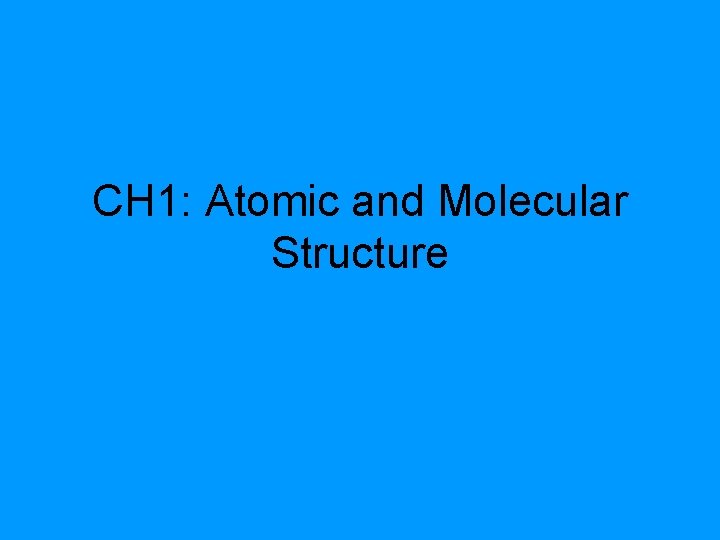
CH 1: Atomic and Molecular Structure

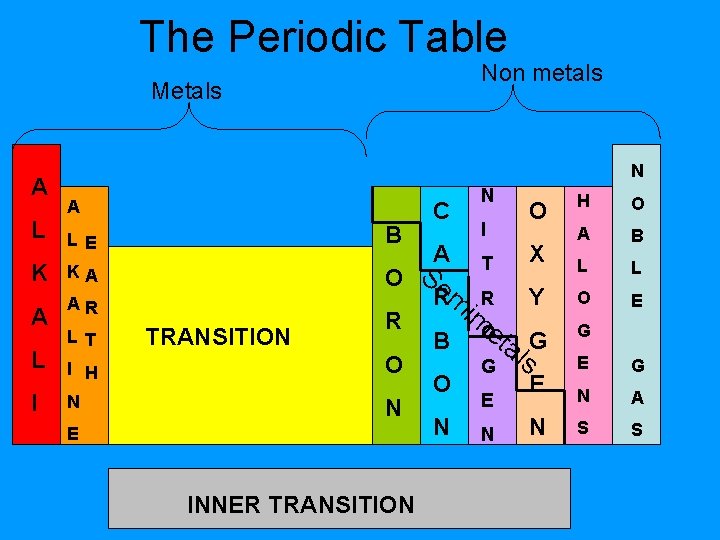
The Periodic Table Non metals Metals A L N A K KA A AR L I B LE LT H O A B L L im. R Y B Oeta G l G s O E E G E N A S S C A O Se TRANSITION R I H O N N E INNER TRANSITION N I T O X Rm O N N E N G


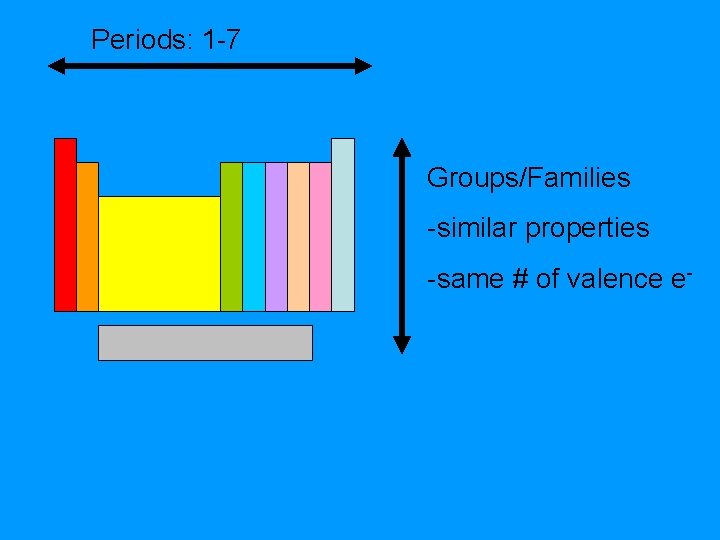
Periods: 1 -7 Groups/Families -similar properties -same # of valence e-

Periodic Table

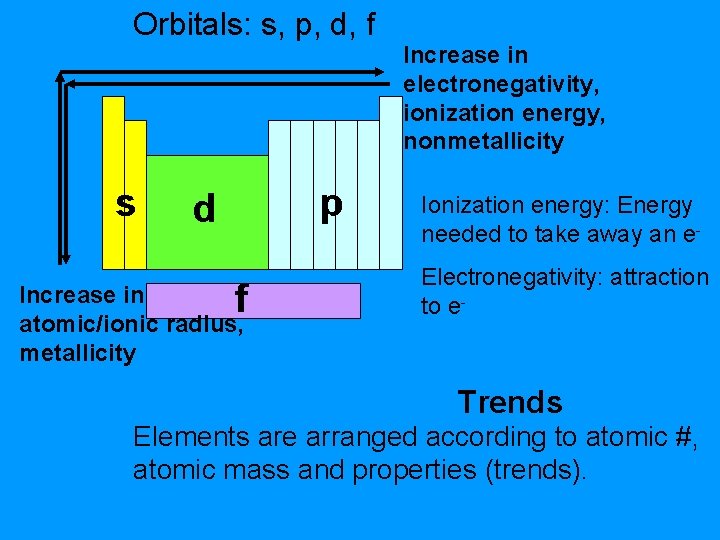
Orbitals: s, p, d, f s p d f Increase in atomic/ionic radius, metallicity Increase in electronegativity, ionization energy, nonmetallicity Ionization energy: Energy needed to take away an e. Electronegativity: attraction to e- Trends Elements are arranged according to atomic #, atomic mass and properties (trends).

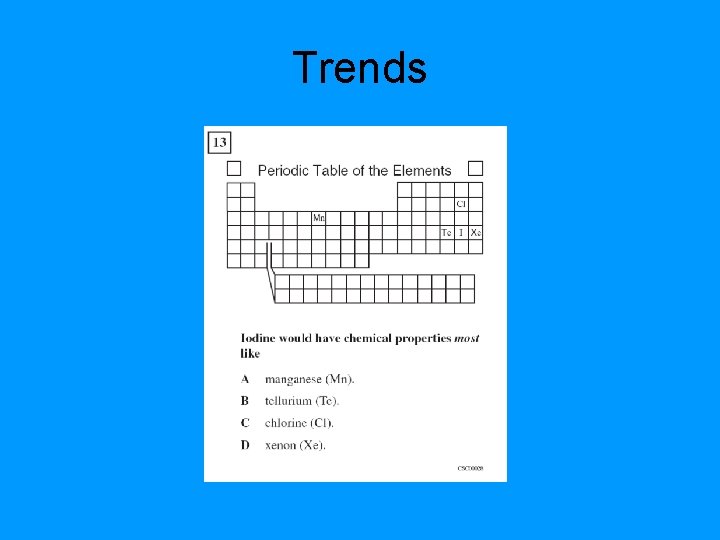
Trends

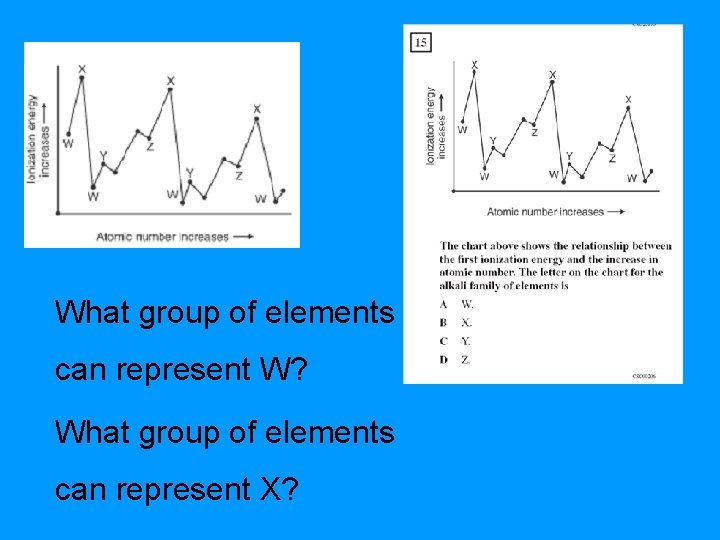
What group of elements can represent W? What group of elements can represent X?

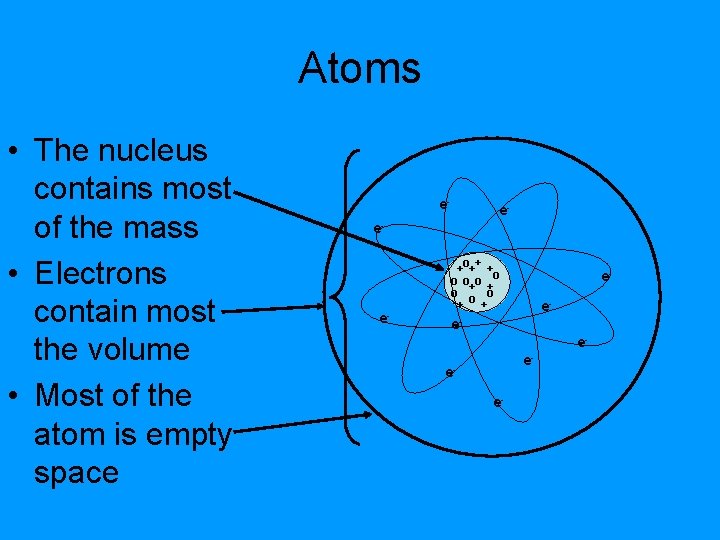
Atoms • The nucleus contains most of the mass • Electrons contain most the volume • Most of the atom is empty space e- e- o+ + + +o o o+o + o o o + + ee- ee-

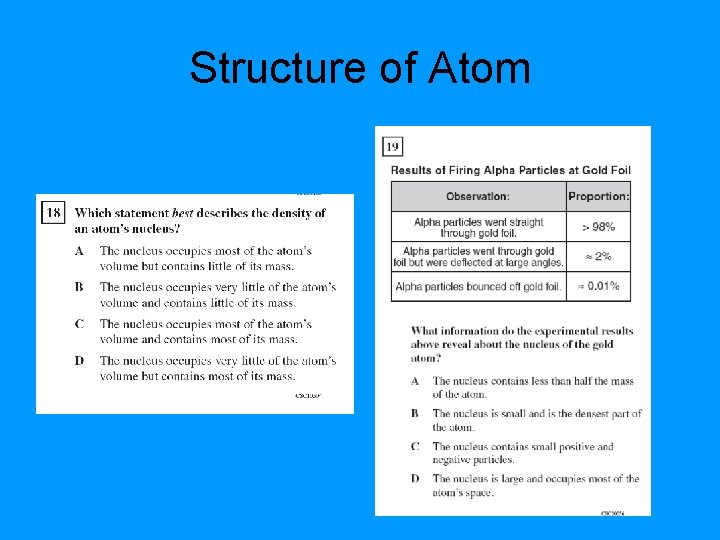
Structure of Atom

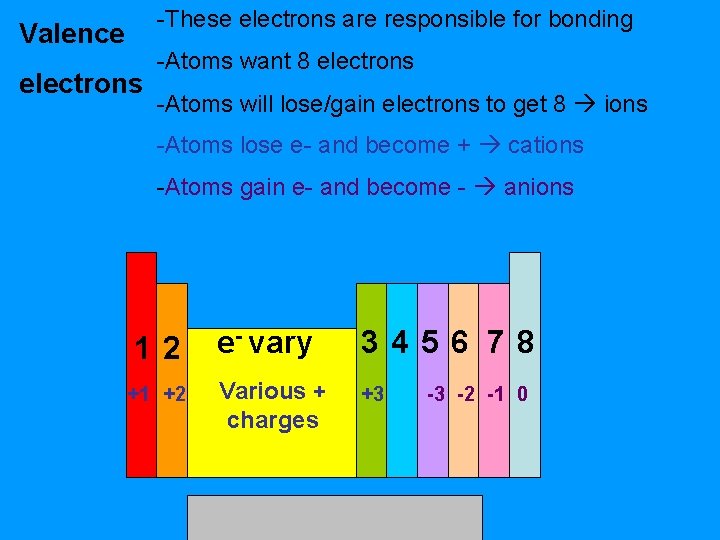
-These electrons are responsible for bonding Valence electrons -Atoms want 8 electrons -Atoms will lose/gain electrons to get 8 ions -Atoms lose e- and become + cations -Atoms gain e- and become - anions 12 +1 +2 e- vary 3456 78 Various + charges +3 -3 -2 -1 0

Valence electrons & trends

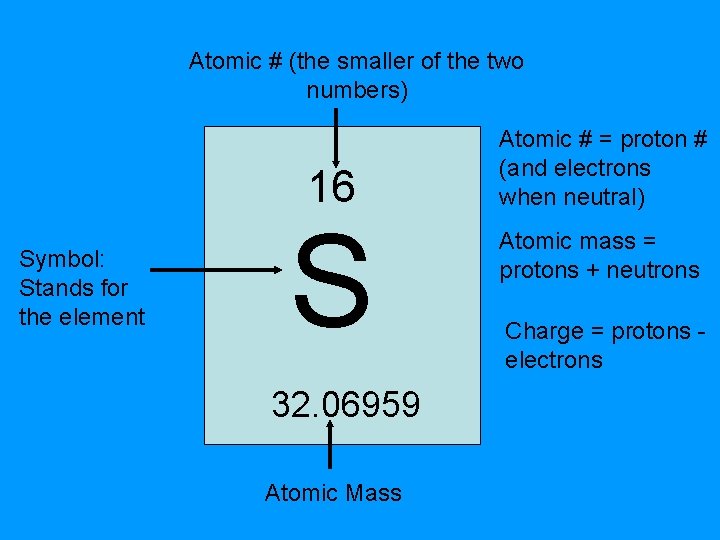
Atomic # (the smaller of the two numbers) 16 Symbol: Stands for the element S 32. 06959 Atomic Mass Atomic # = proton # (and electrons when neutral) Atomic mass = protons + neutrons Charge = protons electrons

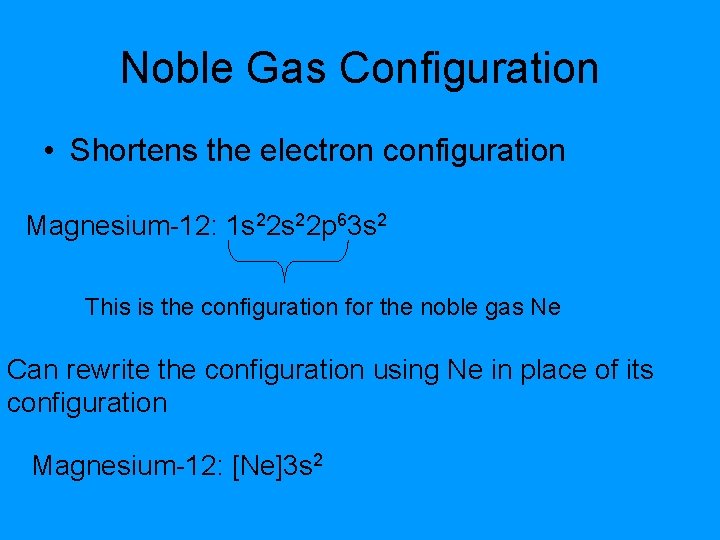
Noble Gas Configuration • Shortens the electron configuration Magnesium-12: 1 s 22 p 63 s 2 This is the configuration for the noble gas Ne Can rewrite the configuration using Ne in place of its configuration Magnesium-12: [Ne]3 s 2

CH 2: Chemical Bonds

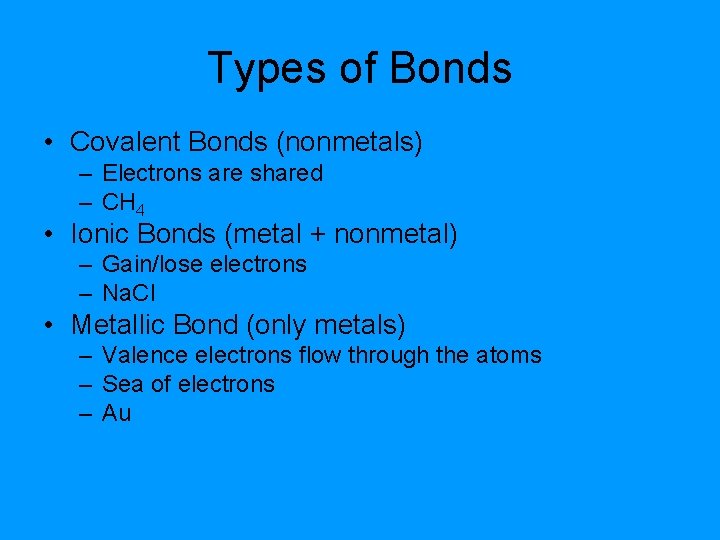
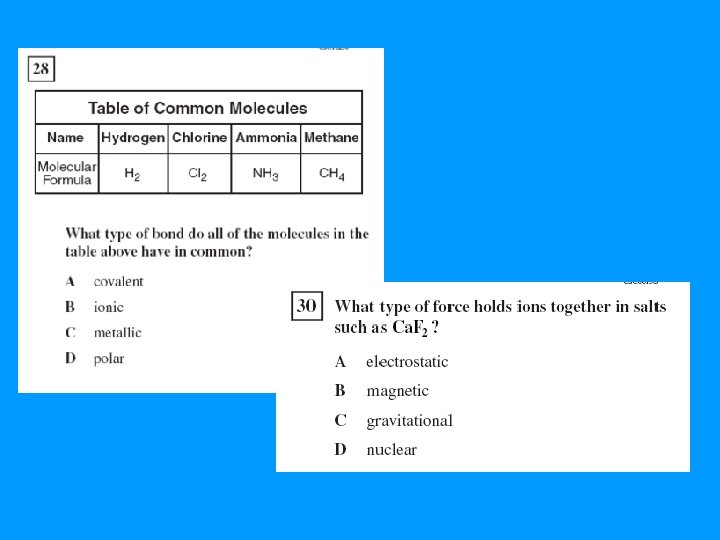
Types of Bonds • Covalent Bonds (nonmetals) – Electrons are shared – CH 4 • Ionic Bonds (metal + nonmetal) – Gain/lose electrons – Na. Cl • Metallic Bond (only metals) – Valence electrons flow through the atoms – Sea of electrons – Au



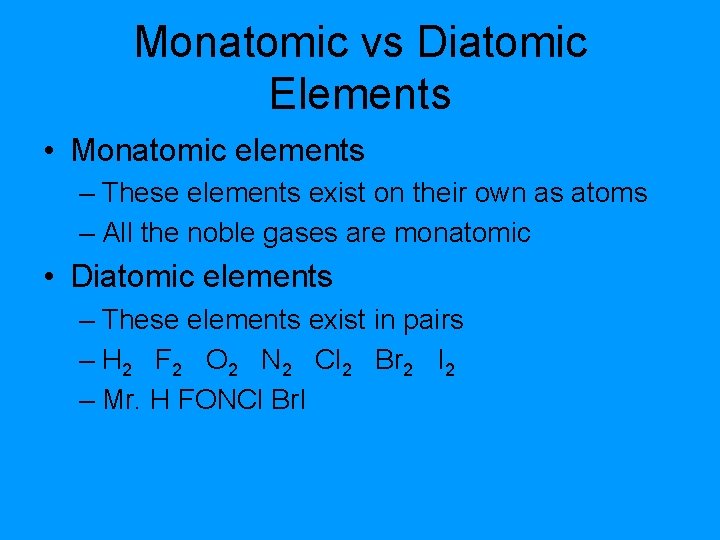
Monatomic vs Diatomic Elements • Monatomic elements – These elements exist on their own as atoms – All the noble gases are monatomic • Diatomic elements – These elements exist in pairs – H 2 F 2 O 2 N 2 Cl 2 Br 2 I 2 – Mr. H FONCl Br. I

H. FONCl Br. I

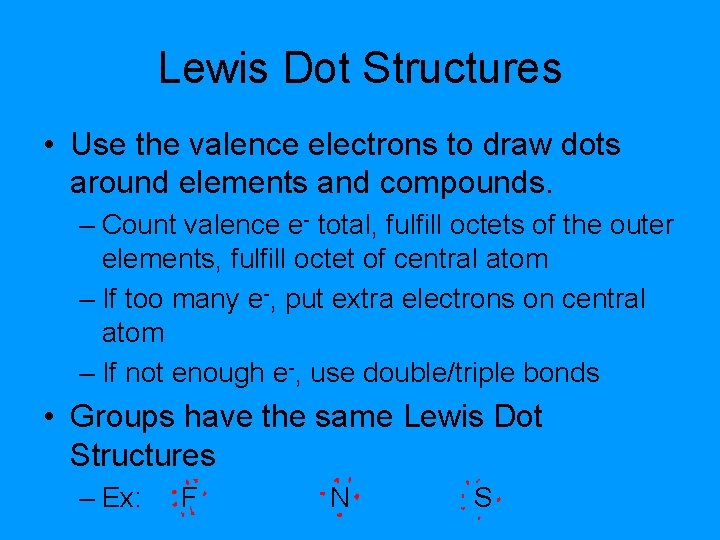
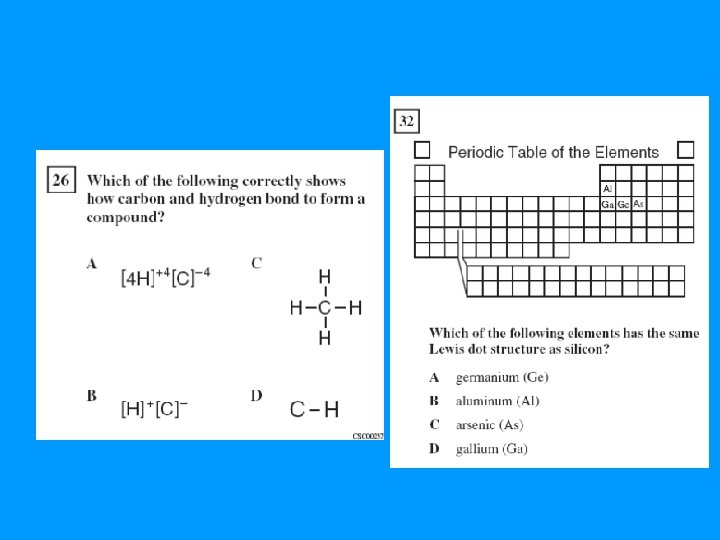
Lewis Dot Structures • Use the valence electrons to draw dots around elements and compounds. – Count valence e- total, fulfill octets of the outer elements, fulfill octet of central atom – If too many e-, put extra electrons on central atom – If not enough e-, use double/triple bonds • Groups have the same Lewis Dot Structures – Ex: F N S

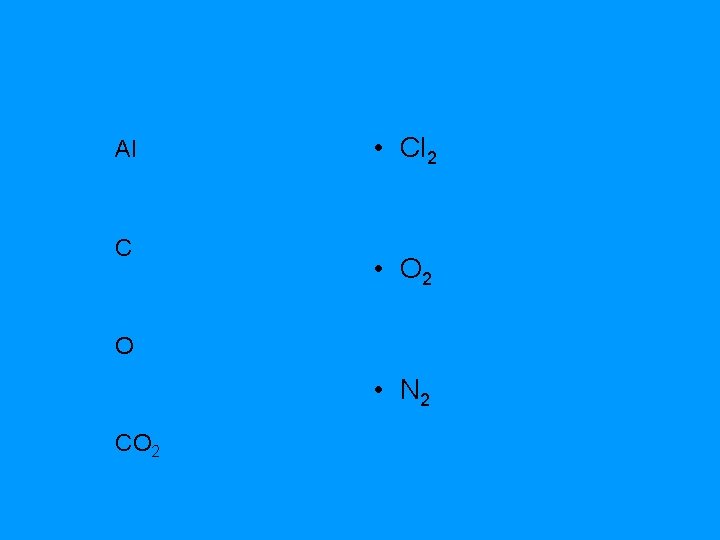
Al C • Cl 2 • O 2 O • N 2 CO 2
