Ch 01 Basic Concepts of Medical Instrumentation 1

Ch 01 Basic Concepts of Medical Instrumentation
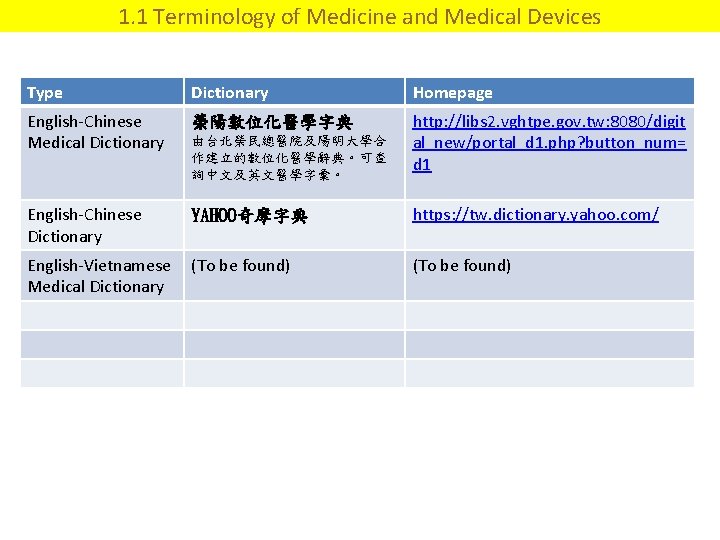
1. 1 Terminology of Medicine and Medical Devices Type Dictionary Homepage English-Chinese Medical Dictionary 榮陽數位化醫學字典 由台北榮民總醫院及陽明大學合 作建立的數位化醫學辭典。可查 詢中文及英文醫學字彙。 http: //libs 2. vghtpe. gov. tw: 8080/digit al_new/portal_d 1. php? button_num= d 1 English-Chinese Dictionary YAHOO奇摩字典 https: //tw. dictionary. yahoo. com/ English-Vietnamese (To be found) Medical Dictionary (To be found)


1. 2 Generalized Medical Instrumentation System

Control And feedback Power source Sensor Measurand Primary Sensing element Variable Conversion element Signal processing (Display, printer, etc. ) (Transducer) Calibration signal Output display Perceptible output Data storage Data transmission Radiation, electric current, or other applied energy Figure 1. 1 Generalized instrumentation system The sensor converts energy or information from the measurand to another form (usually electric). This signal is the processed and displayed so that humans can perceive the information. Elements and connections shown by dashed lines are optional for some applications.
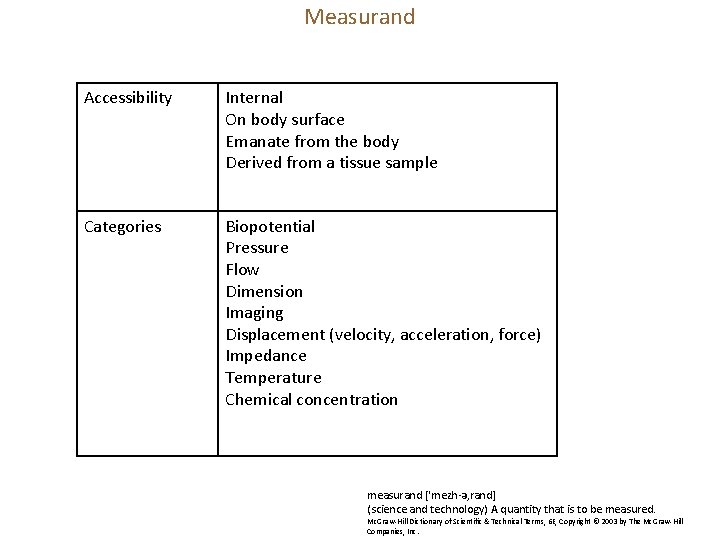
Measurand Accessibility Internal On body surface Emanate from the body Derived from a tissue sample Categories Biopotential Pressure Flow Dimension Imaging Displacement (velocity, acceleration, force) Impedance Temperature Chemical concentration measurand [′mezh·ə‚rand] (science and technology) A quantity that is to be measured. Mc. Graw-Hill Dictionary of Scientific & Technical Terms, 6 E, Copyright © 2003 by The Mc. Graw-Hill Companies, Inc.

1. 3 Alternative Operational Modes Direct-indirect modes Direct: The measurand is accessible. Indirect: The measurand is inaccessible. E. g. : cardiac output (blood volume/min from the heart), morphology of internal organs, pulmonary volume Sampling and continuous modes Can be sampled infrequently: e. g. , body temperature, ion concentration Must be monitored continuously: e. g. , respiratory gas flow, ECG Generating and modulating sensors • Generating sensors: produce their signal output from energy taken directly from the measurand; e. g. , photovoltaic cell * Modulating sensors: use the measurand to alter the flow of energy from an external source in a way that affects the output of the sensor; e. g. , photoconductive cell Analog and digital modes * Analog: able to take on any value within the dynamic range * Digital: accuracy, repeatability, reliability, noise-immunity, not requiring periodic calibration Real-time and delayed-time modes Acquire or display the result in real time: when urgent feedback and control tasks depend on the output Acquire or display the result in delayed time: e. g. cell culture
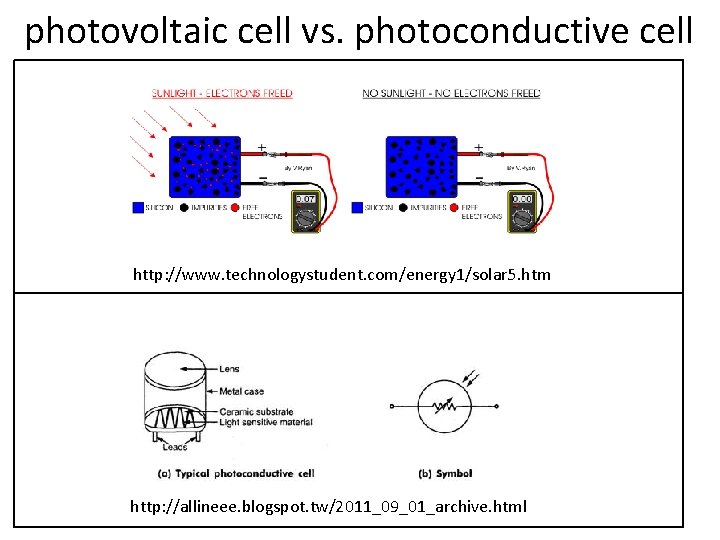
photovoltaic cell vs. photoconductive cell http: //www. technologystudent. com/energy 1/solar 5. htm http: //allineee. blogspot. tw/2011_09_01_archive. html
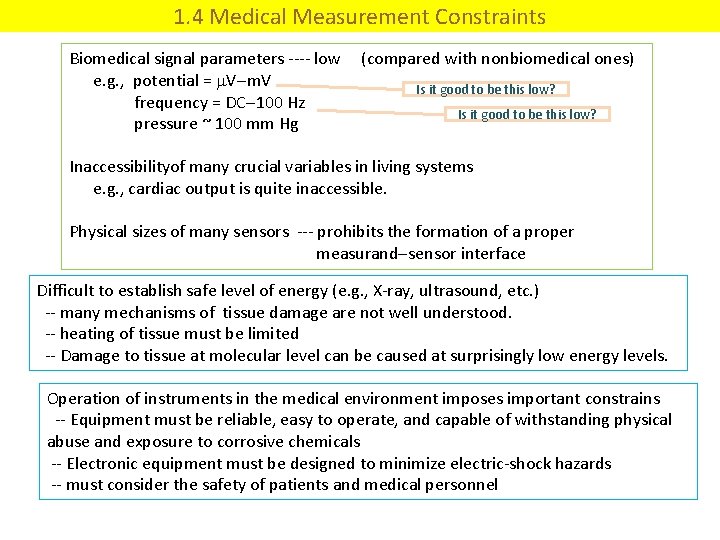
1. 4 Medical Measurement Constraints Biomedical signal parameters ---- low (compared with nonbiomedical ones) e. g. , potential = V m. V Is it good to be this low? frequency = DC 100 Hz Is it good to be this low? pressure ~ 100 mm Hg Inaccessibilityof many crucial variables in living systems e. g. , cardiac output is quite inaccessible. Physical sizes of many sensors --- prohibits the formation of a proper measurand sensor interface Difficult to establish safe level of energy (e. g. , X-ray, ultrasound, etc. ) -- many mechanisms of tissue damage are not well understood. -- heating of tissue must be limited -- Damage to tissue at molecular level can be caused at surprisingly low energy levels. Operation of instruments in the medical environment imposes important constrains -- Equipment must be reliable, easy to operate, and capable of withstanding physical abuse and exposure to corrosive chemicals -- Electronic equipment must be designed to minimize electric-shock hazards -- must consider the safety of patients and medical personnel
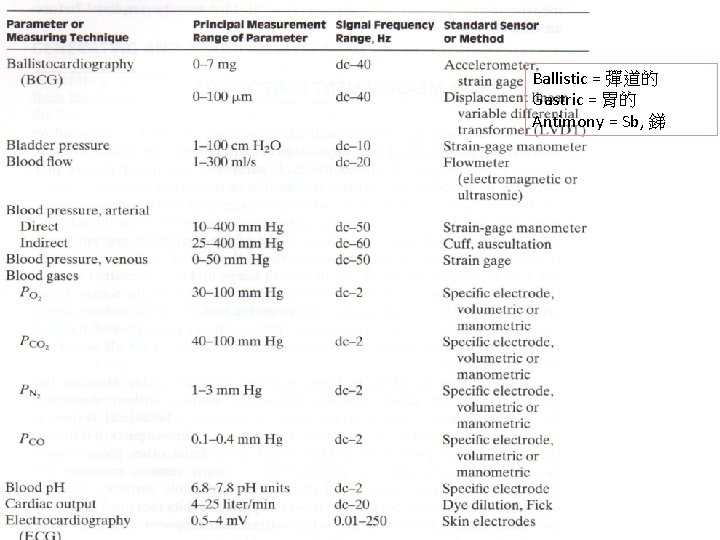
Ballistic = 彈道的 Gastric = 胃的 Antimony = Sb, 銻

a change in the electrical resistance of the skin occurring in moments of strong emotion; measurements of this change are used in lie detector tests GSR galvanic skin response definition Function: : a change in the electrical resistance of the skin that is a physiochemical response to emotional arousal which increases sympathetic nervous system activity — abbreviation GSR Merriam-Webster's Medical Dictionary, © 2007 Merriam-Webster, Inc. Source: Dictionary. com, "galvanic skin response, " in Merriam-Webster's Medical Dictionary. Source location: Merriam-Webster, Inc. http: //dictionary. reference. com/browse/galvanic skin response. Available: http: //dictionary. reference. com. Accessed: February 23, 2011.
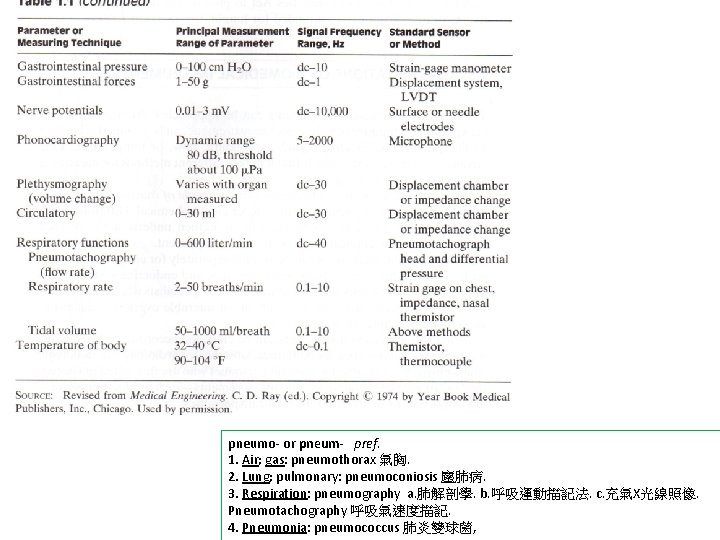
s pneumo- or pneum- pref. 1. Air; gas: pneumothorax 氣胸. 2. Lung; pulmonary: pneumoconiosis 塵肺病. 3. Respiration: pneumography a. 肺解剖學. b. 呼吸運動描記法. c. 充氣X光線照像. Pneumotachography 呼吸氣速度描記. 4. Pneumonia: pneumococcus 肺炎雙球菌,
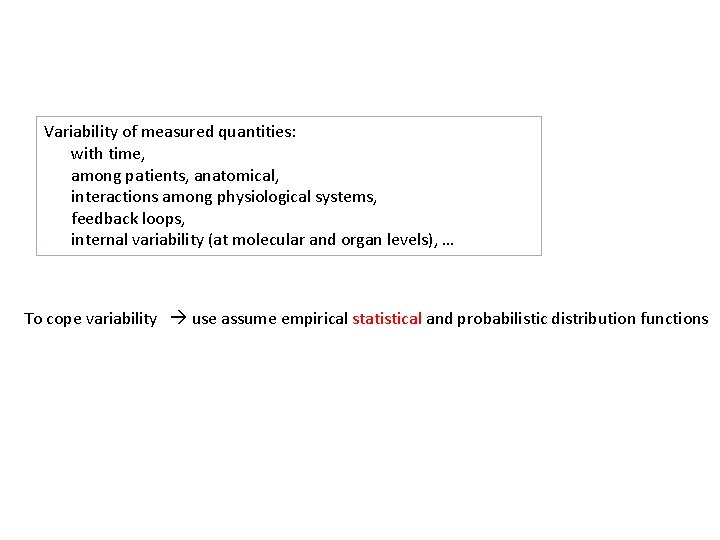
Variability of measured quantities: with time, among patients, anatomical, interactions among physiological systems, feedback loops, internal variability (at molecular and organ levels), … To cope variability use assume empirical statistical and probabilistic distribution functions
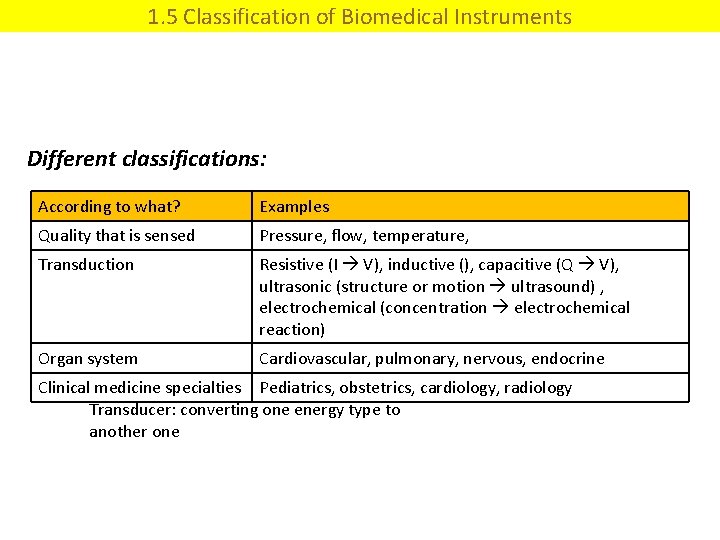
1. 5 Classification of Biomedical Instruments Different classifications: According to what? Examples Quality that is sensed Pressure, flow, temperature, Transduction Resistive (I V), inductive (), capacitive (Q V), ultrasonic (structure or motion ultrasound) , electrochemical (concentration electrochemical reaction) Organ system Cardiovascular, pulmonary, nervous, endocrine Clinical medicine specialties Pediatrics, obstetrics, cardiology, radiology Transducer: converting one energy type to another one

1. 6 Interfering and Modifying Inputs
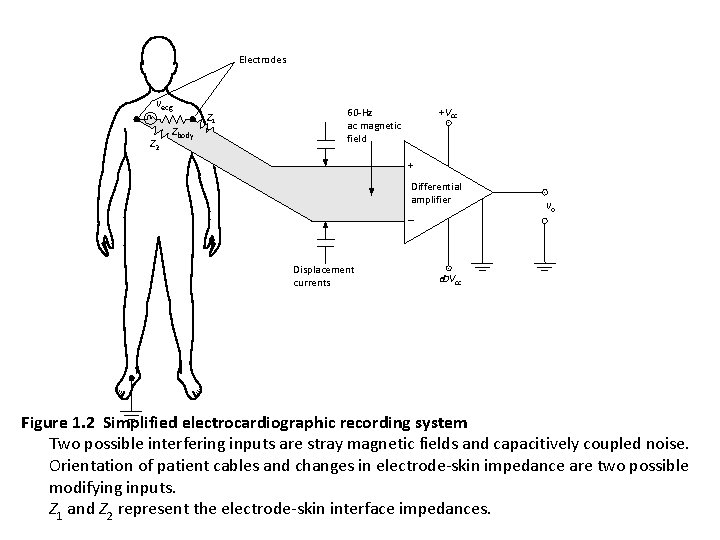
Electrodes vecg Z 2 Zbody Z 1 +Vcc 60 -Hz ac magnetic field + Differential amplifier Displacement currents vo -Vcc Figure 1. 2 Simplified electrocardiographic recording system Two possible interfering inputs are stray magnetic fields and capacitively coupled noise. Orientation of patient cables and changes in electrode-skin impedance are two possible modifying inputs. Z 1 and Z 2 represent the electrode-skin interface impedances.


1. 7 Compensation Techniques How to reduce or eliminate the effects of interfering and modifying inputs
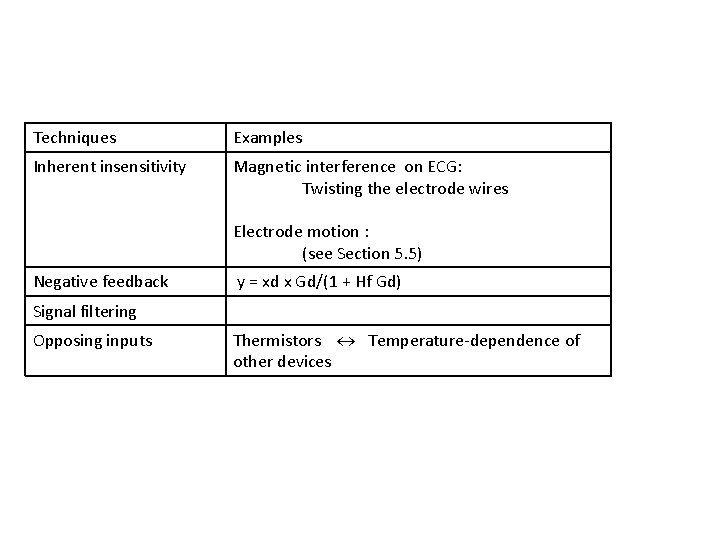
Techniques Examples Inherent insensitivity Magnetic interference on ECG: Twisting the electrode wires Electrode motion : (see Section 5. 5) Negative feedback y = xd x Gd/(1 + Hf Gd) Signal filtering Opposing inputs Thermistors Temperature-dependence of other devices

1. 8 Biostatistics
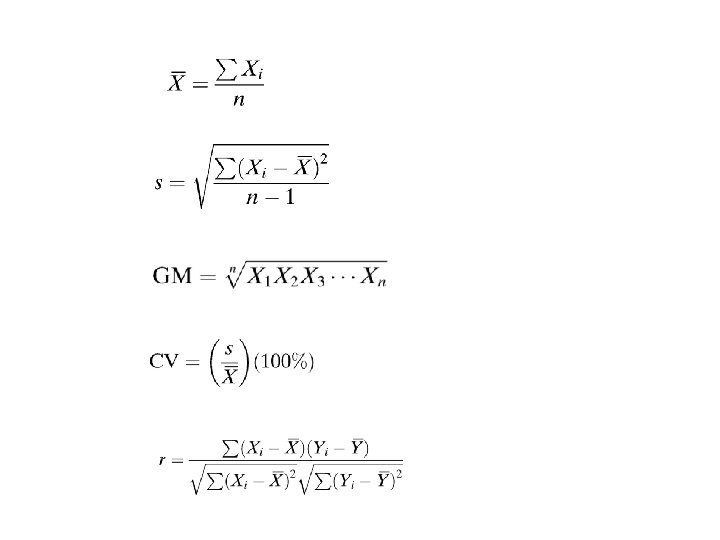
1. 9 Generalized Static Characteristics Accuracy Precision Resolution Reproducibility Statistical control Static sensitivity Zero drift Sensitivity drift Linearity Input range Input impedance To tolerate random variations; By averaging;
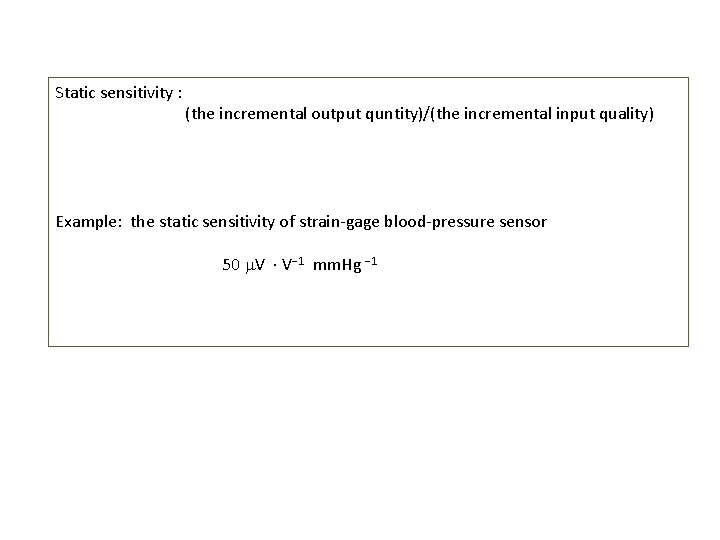
Static sensitivity : (the incremental output quntity)/(the incremental input quality) Example: the static sensitivity of strain-gage blood-pressure sensor 50 V V 1 mm. Hg 1
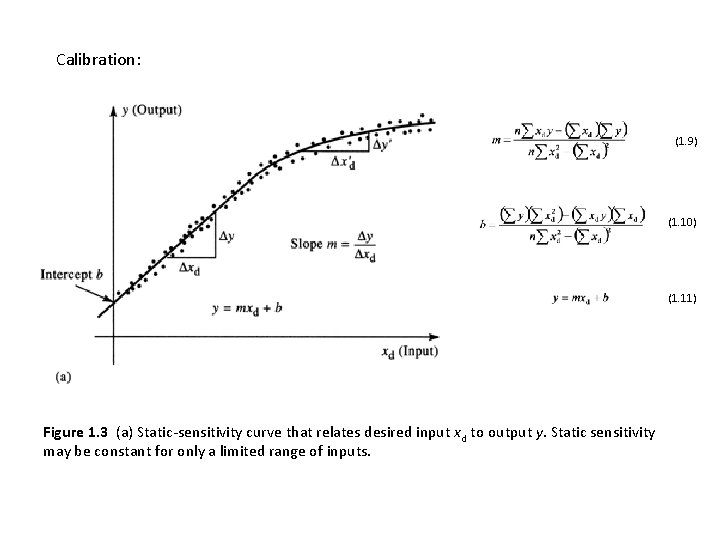
Calibration: (1. 9) y (Output) D x'd Dy Intercept b Slope m = Dxd D y' (1. 10) Dy Dxd y = mxd + b xd (Input) (a) Figure 1. 3 (a) Static-sensitivity curve that relates desired input xd to output y. Static sensitivity may be constant for only a limited range of inputs. (1. 11)
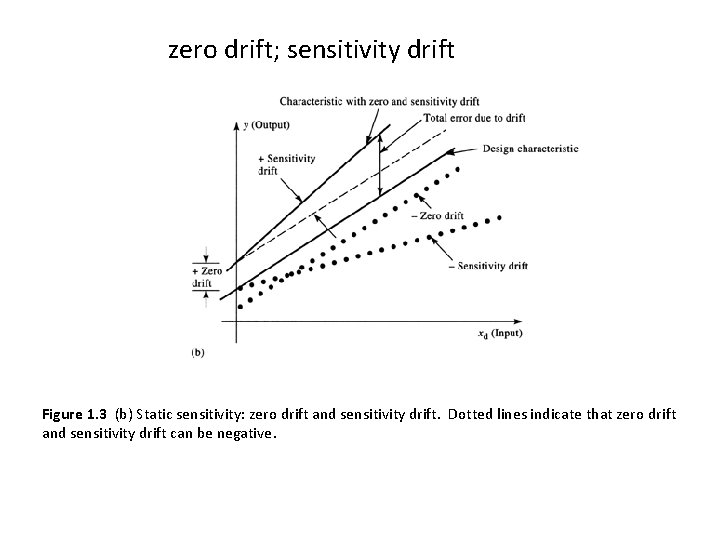
zero drift; sensitivity drift Characteristic with zero and sensitivity drift Total error due to drift y (Output) + Sensitivity drift Zero drift + Zero drift Sensitivity drift xd (Input) (b) Figure 1. 3 (b) Static sensitivity: zero drift and sensitivity drift. Dotted lines indicate that zero drift and sensitivity drift can be negative.
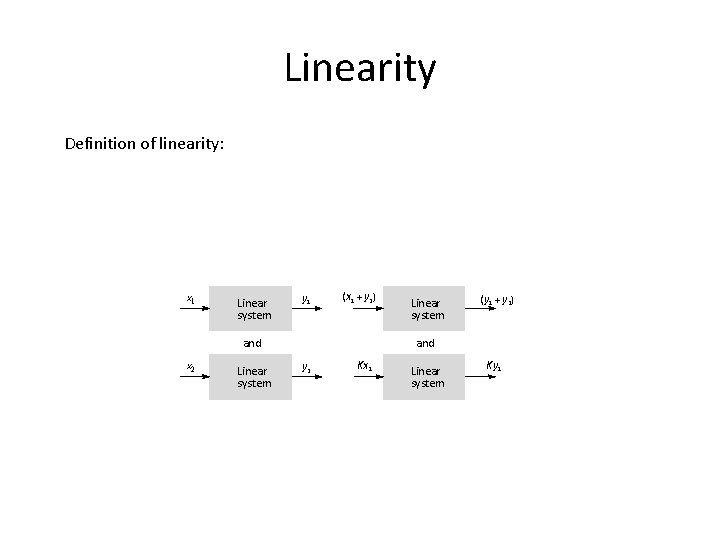
Linearity Definition of linearity: x 1 Linear system y 1 (x 1 + y 2) and x 2 Linear system (y 1 + y 2) and y 2 Kx 1 Linear system Ky 1
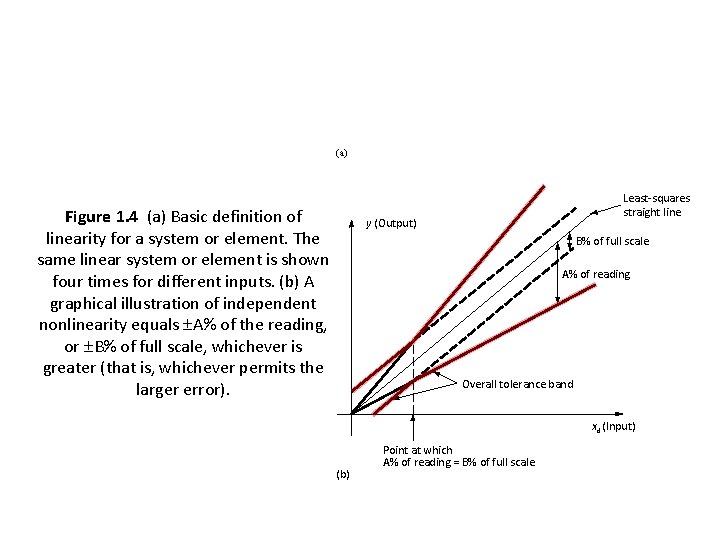
(a) Figure 1. 4 (a) Basic definition of linearity for a system or element. The same linear system or element is shown four times for different inputs. (b) A graphical illustration of independent nonlinearity equals A% of the reading, or B% of full scale, whichever is greater (that is, whichever permits the larger error). Least-squares straight line y (Output) B% of full scale A% of reading Overall tolerance band xd (Input) (b) Point at which A% of reading = B% of full scale
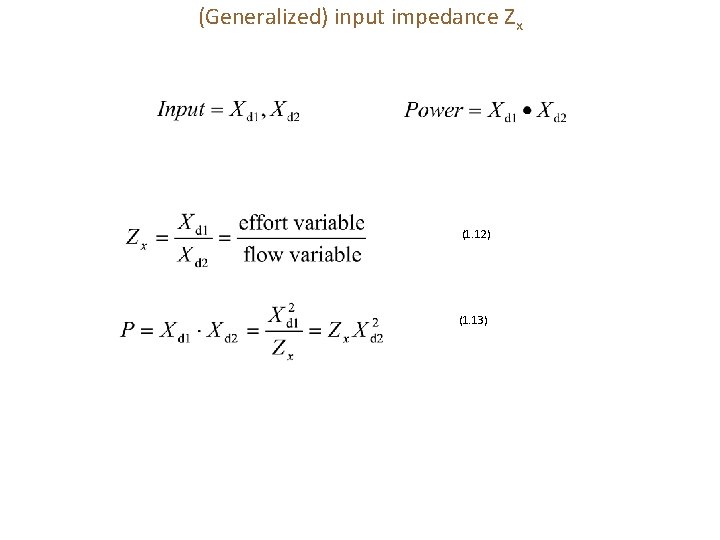
(Generalized) input impedance Zx (1. 12) (1. 13)
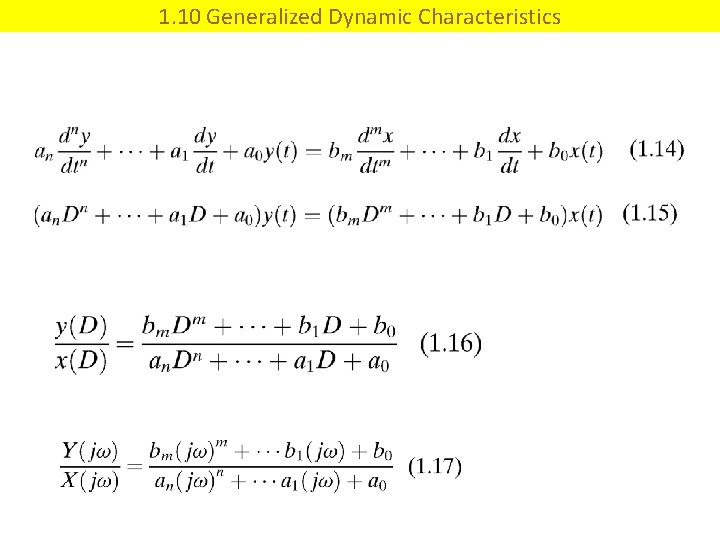
1. 10 Generalized Dynamic Characteristics
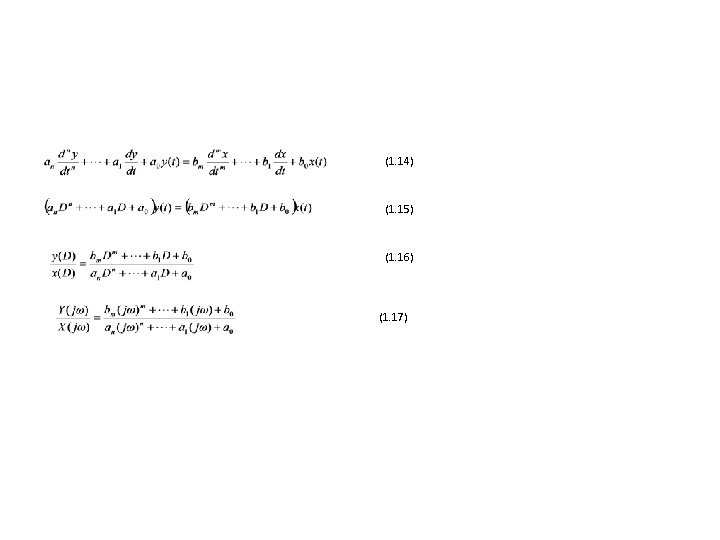
(1. 14) (1. 15) (1. 16) (1. 17)
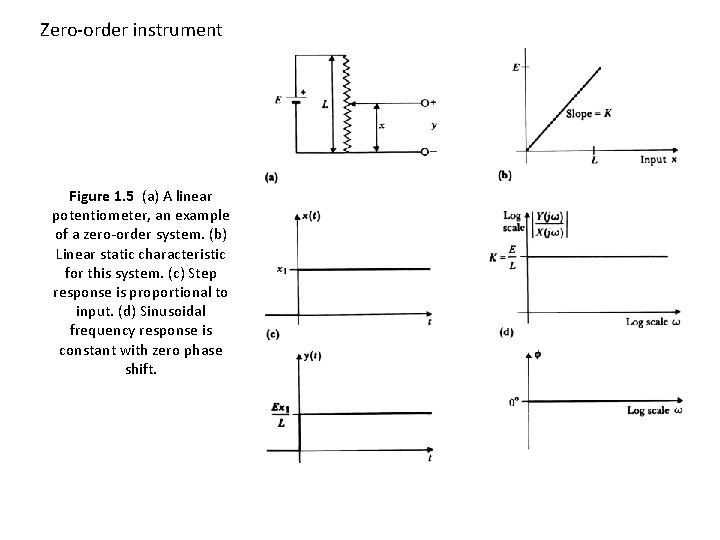
Zero-order instrument Figure 1. 5 (a) A linear potentiometer, an example of a zero-order system. (b) Linear static characteristic for this system. (c) Step response is proportional to input. (d) Sinusoidal frequency response is constant with zero phase shift.
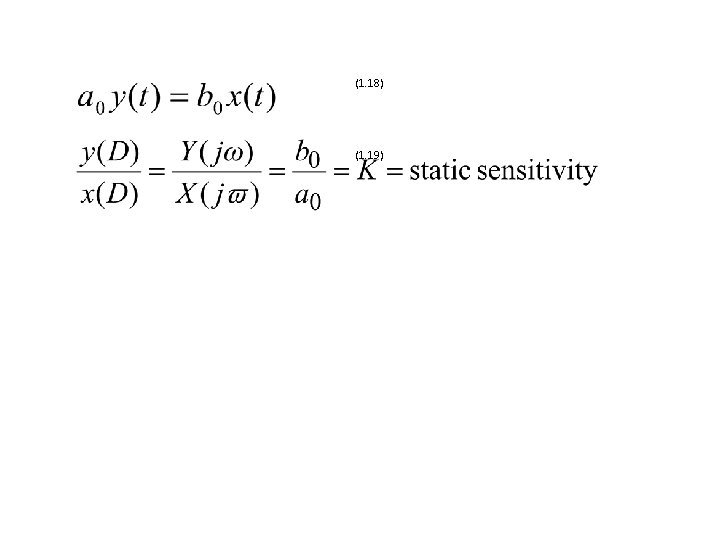
(1. 18) (1. 19)
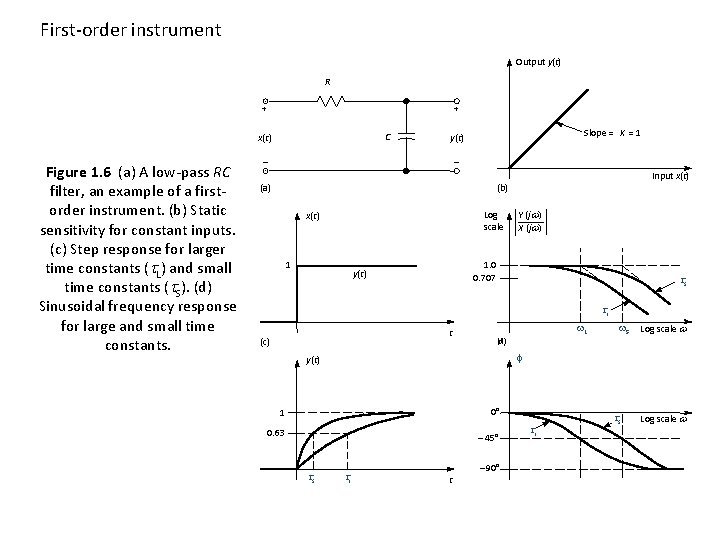
First-order instrument Output y(t) R + + C x(t) Figure 1. 6 (a) A low-pass RC filter, an example of a firstorder instrument. (b) Static sensitivity for constant inputs. (c) Step response for larger time constants ( L) and small time constants ( S). (d) Sinusoidal frequency response for large and small time constants. Slope = K = 1 y(t) (a) Input x(t) (b) Log scale x(t) 1 Y (jw) X (jw) 1. 0 0. 707 y(t) S L t (c) w. L (d) Log scale w f y(t) 0° 1 0. 63 45° S w. S L 90° t L S Log scale w

(1. 20) (1. 21) (1. 22) (1. 23) (1. 24)
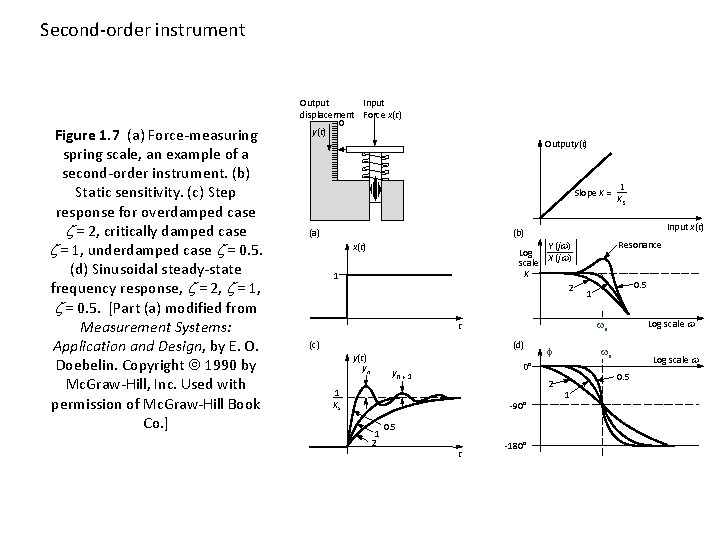
Second-order instrument Figure 1. 7 (a) Force-measuring spring scale, an example of a second-order instrument. (b) Static sensitivity. (c) Step response for overdamped case = 2, critically damped case = 1, underdamped case = 0. 5. (d) Sinusoidal steady-state frequency response, = 2, = 1, = 0. 5. [Part (a) modified from Measurement Systems: Application and Design, by E. O. Doebelin. Copyright 1990 by Mc. Graw-Hill, Inc. Used with permission of Mc. Graw-Hill Book Co. ] Output Input displacement Force x(t) 0 y(t) Outputy(t) Slope K = (a) Input x(t) (b) Y (jw) Log scale X (jw) K 2 x(t) 1 (d) y(t) yn f 2 1 Ks -90° 1 2 0. 5 t 0. 5 1 Log scale w wn 0° yn + 1 Resonance wn t (c) 1 Ks -180° Log scale w 0. 5 1
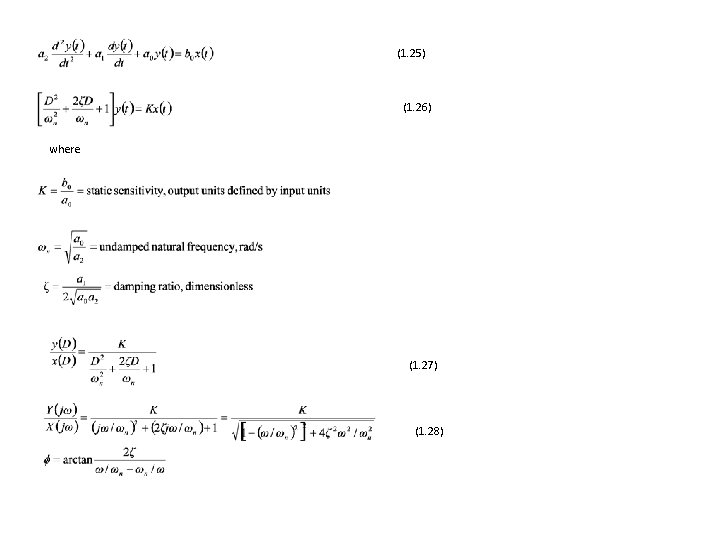
(1. 25) (1. 26) where (1. 27) (1. 28)

(1. 29) (1. 30) (1. 31) (1. 32) Overdamped, (1. 33) Critically damped, (1. 34) Underdamped, (1. 35)
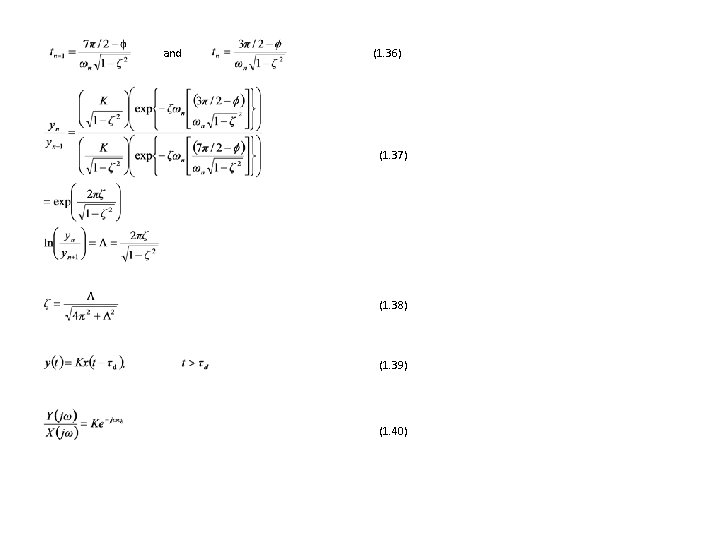
and (1. 36) (1. 37) (1. 38) (1. 39) (1. 40)


1. 11 Design Criteria
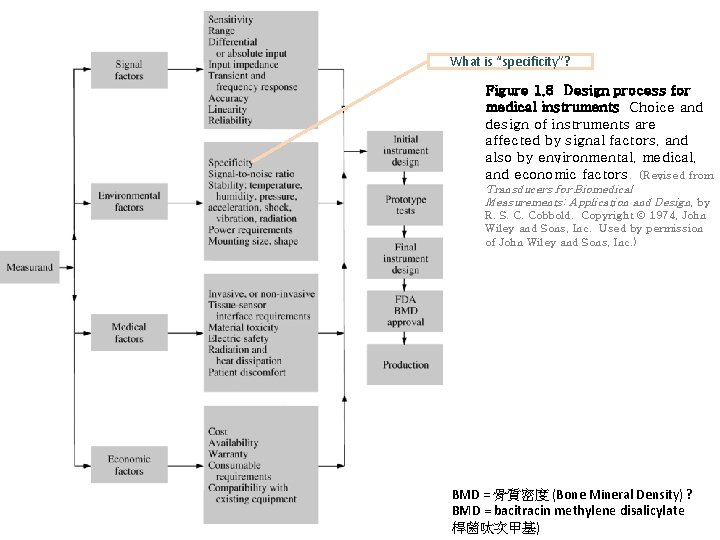
What is “specificity”? Figure 1. 8 Design process for medical instruments Choice and design of instruments are affected by signal factors, and also by environmental, medical, and economic factors. (Revised from Transducers for Biomedical Measurements: Application and Design, by R. S. C. Cobbold. Copyright 1974, John Wiley and Sons, Inc. Used by permission of John Wiley and Sons, Inc. ) BMD = 骨質密度 (Bone Mineral Density) ? BMD = bacitracin methylene disalicylate 桿菌呔次甲基)

1. 12 Commercial Medical Instrumentation Development Process


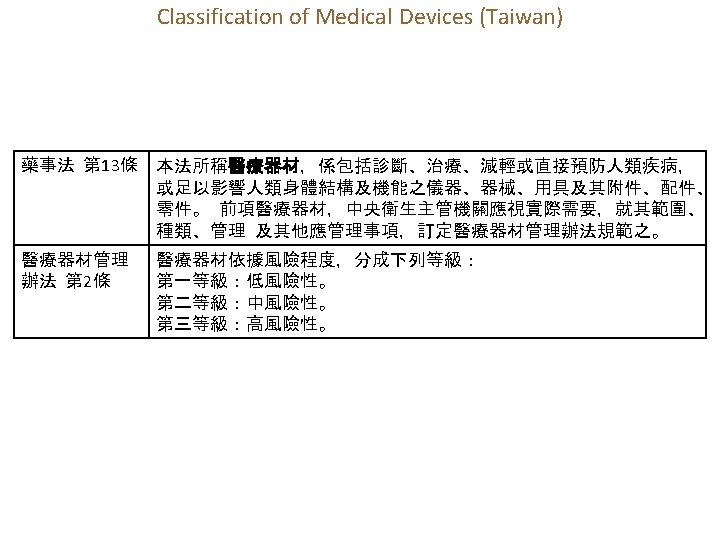
1. 13 Regulation of Medical Devices
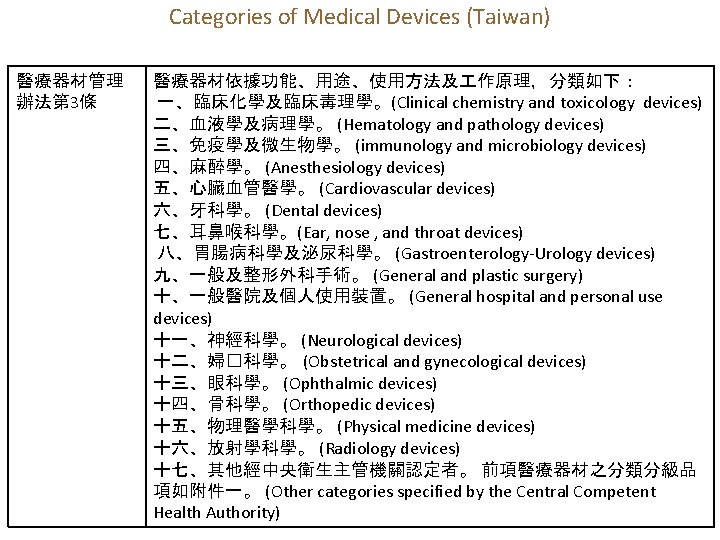
Categories of Medical Devices (Taiwan) 醫療器材管理 辦法第 3條 醫療器材依據功能、用途、使用方法及 作原理,分類如下: 一、臨床化學及臨床毒理學。(Clinical chemistry and toxicology devices) 二、血液學及病理學。 (Hematology and pathology devices) 三、免疫學及微生物學。 (immunology and microbiology devices) 四、麻醉學。 (Anesthesiology devices) 五、心臟血管醫學。 (Cardiovascular devices) 六、牙科學。 (Dental devices) 七、耳鼻喉科學。(Ear, nose , and throat devices) 八、胃腸病科學及泌尿科學。 (Gastroenterology-Urology devices) 九、一般及整形外科手術。 (General and plastic surgery) 十、一般醫院及個人使用裝置。 (General hospital and personal use devices) 十一、神經科學。 (Neurological devices) 十二、婦�科學。 (Obstetrical and gynecological devices) 十三、眼科學。 (Ophthalmic devices) 十四、骨科學。 (Orthopedic devices) 十五、物理醫學科學。 (Physical medicine devices) 十六、放射學科學。 (Radiology devices) 十七、其他經中央衛生主管機關認定者。 前項醫療器材之分類分級品 項如附件一。 (Other categories specified by the Central Competent Health Authority)

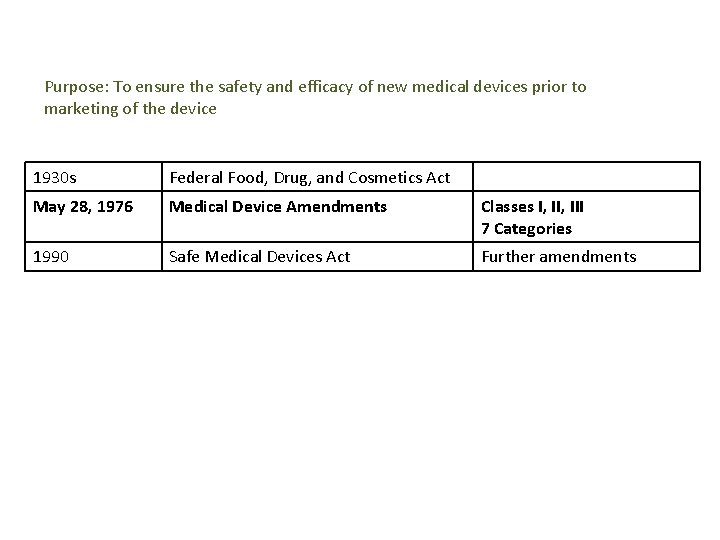
Purpose: To ensure the safety and efficacy of new medical devices prior to marketing of the device 1930 s Federal Food, Drug, and Cosmetics Act May 28, 1976 Medical Device Amendments Classes I, III 7 Categories 1990 Safe Medical Devices Act Further amendments
![“Medical Device” definition by FDA [Source: Wikipedia] Definition in USA by the Food and “Medical Device” definition by FDA [Source: Wikipedia] Definition in USA by the Food and](http://slidetodoc.com/presentation_image_h/059c97d7faada43a7819d5f0d226ee45/image-49.jpg)
“Medical Device” definition by FDA [Source: Wikipedia] Definition in USA by the Food and Drug Administration Medical Device Definition A device is: "an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is: - recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them, - intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or - intended to affect the structure or any function of the body of man or other animals, and which does not achieve any of it's primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes. Contrivance 發明物; 裝置, 器械[C] Formulary 公式的, 規定的 Pharmacopoeia 藥典; 配藥書
![Medical Device (defined by FDA) [Source FDA website] Medical Device Definition Medical devices range Medical Device (defined by FDA) [Source FDA website] Medical Device Definition Medical devices range](http://slidetodoc.com/presentation_image_h/059c97d7faada43a7819d5f0d226ee45/image-50.jpg)
Medical Device (defined by FDA) [Source FDA website] Medical Device Definition Medical devices range from simple tongue depressors and bedpans to complex programmable pacemakers with micro-chip technology and laser surgical devices. In addition, medical devices include in vitro diagnostic products, such as general purpose lab equipment, reagents, and test kits, which may include monoclonal antibody technology. Certain electronic radiation emitting products 3 with medical application and claims meet the definition of medical device. Examples include diagnostic ultrasound products, x-ray machines and medical lasers. If a product is labeled, promoted or used in a manner that meets the following definition in section 201(h) of the Federal Food Drug & Cosmetic (FD&C) Act it will be regulated by the Food and Drug Administration (FDA)4 as a medical device and is subject to premarketing and postmarketing regulatory controls. Bedpan 便盆
![Medical Device (defined by FDA) [Source FDA website] Medical Device Definition A device is: Medical Device (defined by FDA) [Source FDA website] Medical Device Definition A device is:](http://slidetodoc.com/presentation_image_h/059c97d7faada43a7819d5f0d226ee45/image-51.jpg)
Medical Device (defined by FDA) [Source FDA website] Medical Device Definition A device is: "an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is: recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them, intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or intended to affect the structure or any function of the body of man or other animals, and which does not achieve any of it's primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes. " This definition provides a clear distinction between a medical device and other FDA regulated products such as drugs. If the primary intended use of the product is achieved through chemical action or by being metabolized by the body, the product is usually a drug. Human drugs are regulated by FDA‘s Center for Drug Evaluation and Research 5 (CDER). Biological products which include blood and blood products, and blood banking equipment are regulated by FDA’s Center for Biologics Evaluation and Research 6 (CBER). FDA‘s Center for Veterinary Medicine 7 (CVM) regulates products used with animals. If your product is not a medical device but regulated by another Center in the FDA, each component of the FDA has an office to assist with questions about the products they regulate. In cases where it is not clear whether a product is a medical device there are procedures in place to use DSMICA Staff Directory 8 to assist you in making a determination.
![FDA Classification [Source: Wikipedia] United States The Food and Drug Administration has recognized three FDA Classification [Source: Wikipedia] United States The Food and Drug Administration has recognized three](http://slidetodoc.com/presentation_image_h/059c97d7faada43a7819d5f0d226ee45/image-52.jpg)
FDA Classification [Source: Wikipedia] United States The Food and Drug Administration has recognized three classes of medical devices based on the level of control necessary to assure the safety and effectiveness of the device. [7] The classification procedures are described in the Code of Federal Regulations, Title 21, part 860 (usually known as 21 CFR 860). [8] Class I: General controls • Class I devices are subject to the least regulatory control. • Class I devices are subject to “General Controls” as are Class II and Class III devices. General controls include provisions that relate to adulteration; misbranding; device registration and listing; premarket notification; banned devices; notification, including repair, replacement, or refund; records and reports; restricted devices; and good manufacturing practices. • Class I devices are not intended for use in supporting or sustaining life or to be of substantial importance in preventing impairment to human health, and they may not present a potential unreasonable risk of illness or injury. • Most Class I devices are exempt from the premarket notification and/or good manufacturing practices regulation. • Examples of Class I devices include elastic bandages, examination gloves, and hand-held surgical instruments. Adulteration 粗劣品; 攙假貨
![FDA Classification (cont. ) [Source: Wikipedia] Class II: General controls with special controls • FDA Classification (cont. ) [Source: Wikipedia] Class II: General controls with special controls •](http://slidetodoc.com/presentation_image_h/059c97d7faada43a7819d5f0d226ee45/image-53.jpg)
FDA Classification (cont. ) [Source: Wikipedia] Class II: General controls with special controls • Class II devices are those for which general controls alone are insufficient to assure safety and effectiveness, and existing methods are available to provide such assurances. • In addition to complying with general controls, Class II devices are also subject to special controls. [9] A few Class II devices are exempt from the premarket notification. [9] Special controls may include special labeling requirements, mandatory performance standards and postmarket surveillance. [9] • Devices in Class II are held to a higher level of assurance than Class I devices, and are designed to perform as indicated without causing injury or harm to patient or user. • Examples of Class II devices include powered wheelchairs, infusion pumps, and surgical drapes. [7][9] Drape 簾 Class III: general controls and premarket approval • A Class III device is one for which insufficient information exists to assure safety and effectiveness solely through the general or special controls sufficient for Class II devices. [7][9] • Such a device needs premarket approval, a scientific review to ensure the device's safety and effectiveness, in addition to the general controls of Class I. [7][9] • Class III devices are usually those that support or sustain human life, are of substantial importance in preventing impairment of human health, or which present a potential, unreasonable risk of illness or injury. [9] • Examples of Class III devices which currently require a premarket notification include implantable pacemaker, pulse generators, HIV diagnostic tests, automated external Endosseous adj refers to any object, such as [9] defibrillators, and endosseous (placed or contained within a bone) implants. dental implant, placed or contained within a
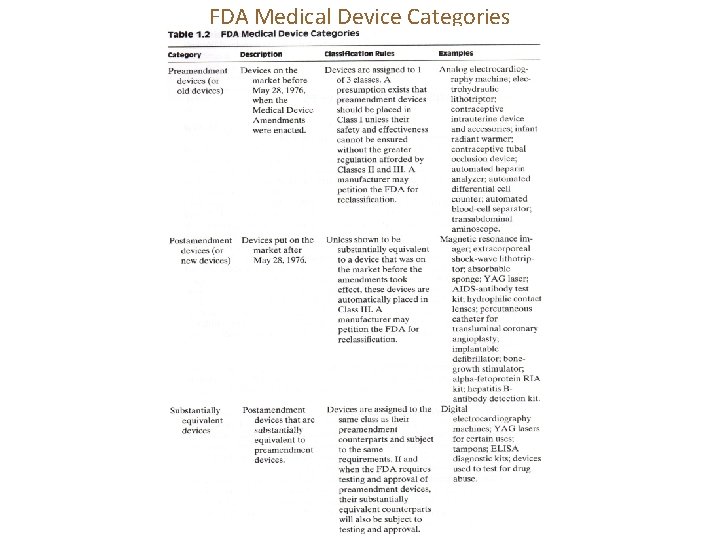
FDA Medical Device Categories
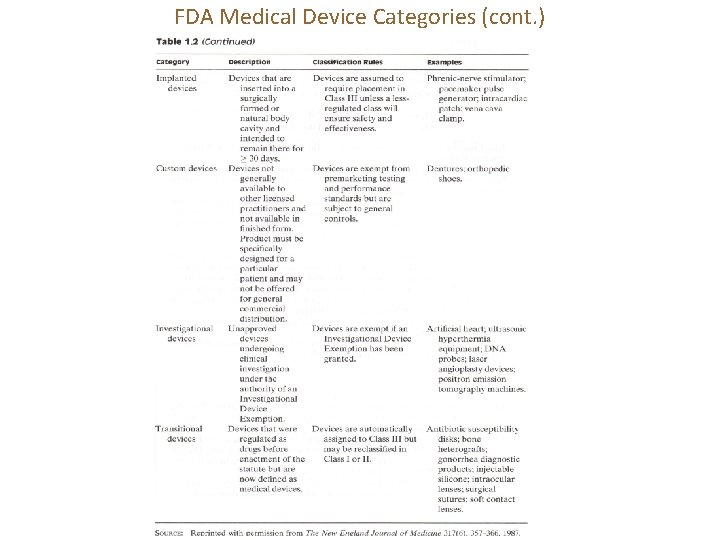
FDA Medical Device Categories (cont. )
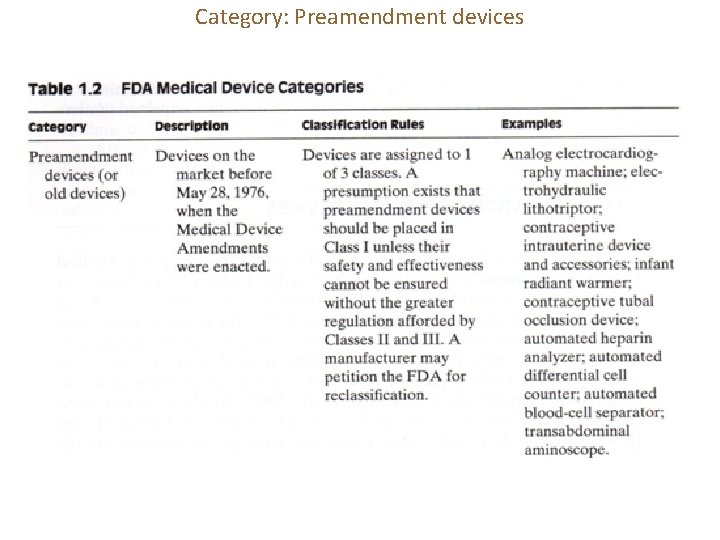
Category: Preamendment devices

Category: Postamendment devices Fetoprotein 胎蛋白; radioimmunoassay (RIA) 放射免疫測定 Extracorporeal shock wave lithotripsy (ESWL) is the non-invasive treatment of kidney stones (urinary calculosis) and biliary calculi (stones in the gallbladder or in the liver) using an acoustic pulse. (Source: Wikipedia) Radioimmunoassay (RIA), an in vitro nuclear medicine, is a very sensitive technique used to measure concentrations of antigens (for example, hormone levels in the blood) by use of antibodies. (Source: Wikipedia)

Category: Substantially equivalent devices Tampon 止血棉球, 月經棉塞; ELISA (Enzyme-linked immunosorbent assay) 酵素連結免 疫吸附法;
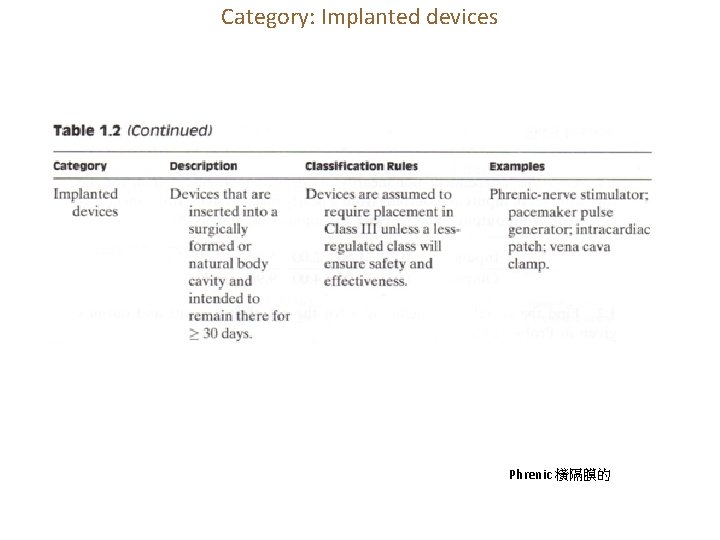
Category: Implanted devices Phrenic 橫隔膜的
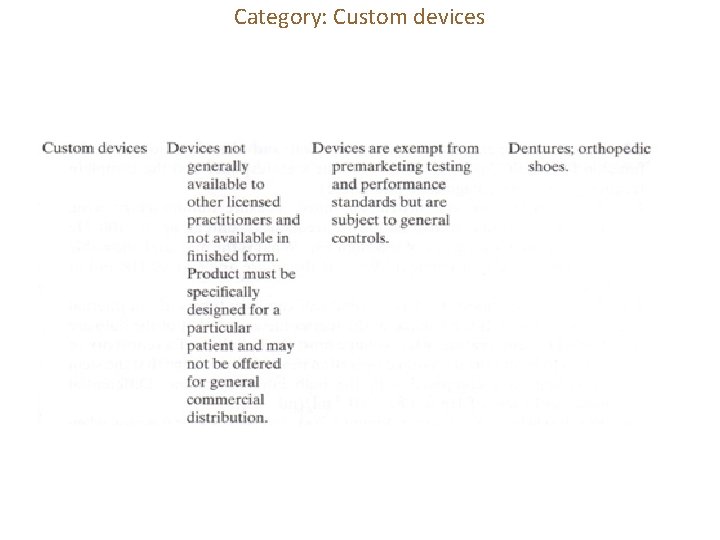
Category: Custom devices

Category: Investigational devices

Category: Transitional devices Transitional 過渡性的; Gonorrhea 淋病; heterograft 異種移植片 An intraocular lens (IOL) is an implanted lens in the eye, usually replacing the existing crystalline lens because it has been clouded over by a cataract, or as a form of refractive surgery to change the eye‘s optical power. [Source: Wikipedia]

From 3 rd Edition

(xd – Hfy)Gd = y (1. 1) xd. Gd = y(1 + Hf. Gd) (1. 2) (1. 3) (1. 4) (1. 5) (1. 6) (1. 7) (1. 8)
- Slides: 64