Certain physical properties associated with certain space groups



![SECOND HANDOUT Refutation of a Claimed Exception to Zink’s Rule: (TEA)[Eu(DBM)4] redetermine I 2/a SECOND HANDOUT Refutation of a Claimed Exception to Zink’s Rule: (TEA)[Eu(DBM)4] redetermine I 2/a](https://slidetodoc.com/presentation_image/6868f0648546b9c19ebea73d75894910/image-7.jpg)
![Three Genuine Exceptions to Zink’s Rule: Structural Redeterminations (piperidinium)[Eu(BA)4] [Tb. Cl 2(H 2 O)6]Cl Three Genuine Exceptions to Zink’s Rule: Structural Redeterminations (piperidinium)[Eu(BA)4] [Tb. Cl 2(H 2 O)6]Cl](https://slidetodoc.com/presentation_image/6868f0648546b9c19ebea73d75894910/image-8.jpg)
![Not Every Noncentrosymmetric Eu(III) Complexes Are Observably Triboluminescent Not Triboluminescent Na 3[Eu(ODA)3] • 8 Not Every Noncentrosymmetric Eu(III) Complexes Are Observably Triboluminescent Not Triboluminescent Na 3[Eu(ODA)3] • 8](https://slidetodoc.com/presentation_image/6868f0648546b9c19ebea73d75894910/image-9.jpg)
- Slides: 9

Certain physical properties associated with certain space groups or point groups · Piezoelectricity is the property of a crystal to develop an electric charge when subjected to pressure or tension in certain directions and is observed in crystals with polar axes. o Applications of piezoelectricity include (but are not limited to) quartz timepieces and microphones; triboluminescence…. later · Optical activity refers to the ability of crystals to rotate planepolarized light. · Pyroelectricity is the property of a crystal to develop electrical polarization when the temperature is changed and can only occur in non-centrosymmetric crystal systems. · Ferroelectricity (important in electro-ceramics) also shows polarization for a temperature change but has the additional feature of changing the direction of polarization in an electric field. See Table below for a listing of the point groups that exhibit the abovementioned properties


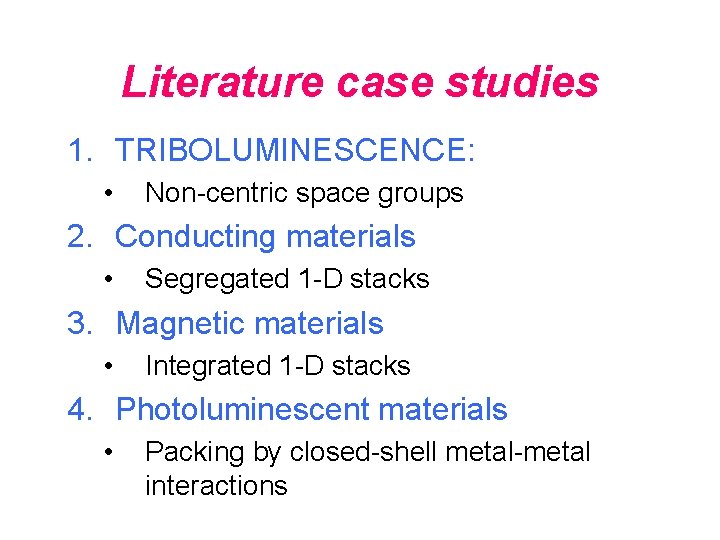
Literature case studies 1. TRIBOLUMINESCENCE: • Non-centric space groups 2. Conducting materials • Segregated 1 -D stacks 3. Magnetic materials • Integrated 1 -D stacks 4. Photoluminescent materials • Packing by closed-shell metal-metal interactions

A Structural Study of Triboluminescence (TL) • General – Almost exclusively seen in crystalline samples – Luminescent species • the surrounding gas (N 2) • the compound itself • a combination of both 11 e. V ground state TL spectrum of sucrose Energy level of N 2 excitation piezoelectricity emission Noncentrosymmetric space group

• Zink’s “golden rule” : only noncentrosymmetric crystals could be triboluminescent. See ABSTRACT & TABLE 1 IN HANDOUT: B. P. Chandra, J. I. Zink, Inorg. Chem. , 1980, 19, 3098. Some other papers by Professor Zink about triboluminescence: • Zink, J. ; Hardy, G. E. ; Sutton, J. E. , J. Phys. Chem. , 1976, 80, No. 3, 248. • G. E. Hardy; J. I. Zink, Inorg. Chem. , 1976, 15, 3061. • B. P. Chandra, J. I. Zink, J. Phys. Chem. , 1982, 86, 4138

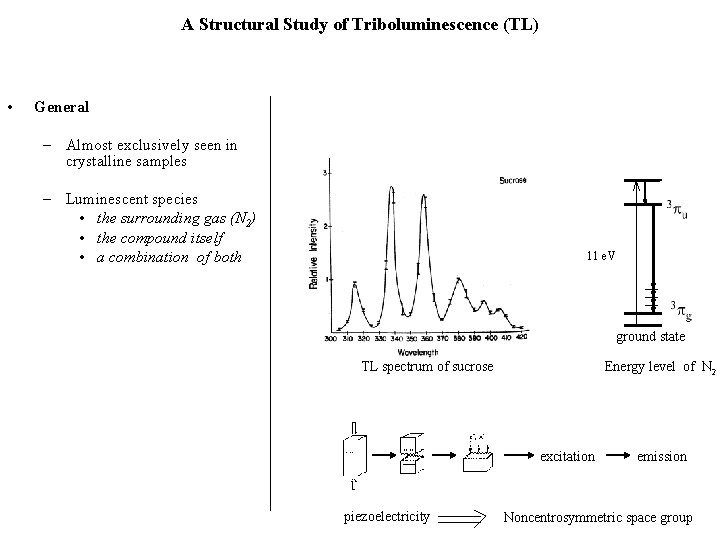
Zink’s Rule is Valid for 11 Tetrahedral Mn(II) Complexes Compound Triboluminescent Space Group Known Previously (Py. H)2 Mn. Cl 4 No P 1 (Me 3 Ph. P)2 Mn. Cl 4 Yes P 213 (Me 4 N)2 Mn. Cl 4 No P 21/n (Et 4 N)2 Mn. Br 4 Yes P 421 m (noncentro) (Bu 4 N)2 Mn. I 4 No (Ph 3 PO)2 Mn. Cl 2 Yes (80 K) (Ph 3 PO)2 Mn. Br 2 Yes P 1 (noncentro) (Ph 3 PO)2 Mn. I 2 Yes P 1 (Ph 3 As. O)2 Mn. Cl 2 No P 21/n (Ph 3 As. O)2 Mn. Br 2 Yes (80 K) Pca 21 (noncentro) (Ph 3 As. O)2 Mn. I 2 No P 1 Fdd 2 Space Group Determined in This Work (noncentro) Cotton, F. A. ; Daniels, L. M. ; Huang, P. Inorg. Chem. 2001, 40, 3576.
![SECOND HANDOUT Refutation of a Claimed Exception to Zinks Rule TEAEuDBM4 redetermine I 2a SECOND HANDOUT Refutation of a Claimed Exception to Zink’s Rule: (TEA)[Eu(DBM)4] redetermine I 2/a](https://slidetodoc.com/presentation_image/6868f0648546b9c19ebea73d75894910/image-7.jpg)
SECOND HANDOUT Refutation of a Claimed Exception to Zink’s Rule: (TEA)[Eu(DBM)4] redetermine I 2/a Z = 4 R(F) = 0. 0749 R(w. F) = 0. 0817 Sweeting, L. M. ; Rheingold, A. L. J. Am. Chem. Soc. , 1987, 109, 2652. Ia Z=4 R 1 = 0. 0581 w. R 2 = 0. 0952 Flack x = 0. 00(2) Cotton, F. A. ; Daniels, L. M. ; Huang, P. Inorg. Chem. Comm. , 2001, 4, 319.
![Three Genuine Exceptions to Zinks Rule Structural Redeterminations piperidiniumEuBA4 Tb Cl 2H 2 O6Cl Three Genuine Exceptions to Zink’s Rule: Structural Redeterminations (piperidinium)[Eu(BA)4] [Tb. Cl 2(H 2 O)6]Cl](https://slidetodoc.com/presentation_image/6868f0648546b9c19ebea73d75894910/image-8.jpg)
Three Genuine Exceptions to Zink’s Rule: Structural Redeterminations (piperidinium)[Eu(BA)4] [Tb. Cl 2(H 2 O)6]Cl P 21/n Z = 4 P 2/n R 1 = 0. 0573 WR 2 = 0. 0887 R 1 = 0. 0147 w. R 2 = 0. 0317 Z = 2 Rheingold, A. L. ; King, W. , Inorg. Chem. , 1989, 28, 1715. [Tb(antipyrine)6]I 3 R Z = 3 R 1 = 0. 0253 WR 2 = 0. 0601
![Not Every Noncentrosymmetric EuIII Complexes Are Observably Triboluminescent Not Triboluminescent Na 3EuODA3 8 Not Every Noncentrosymmetric Eu(III) Complexes Are Observably Triboluminescent Not Triboluminescent Na 3[Eu(ODA)3] • 8](https://slidetodoc.com/presentation_image/6868f0648546b9c19ebea73d75894910/image-9.jpg)
Not Every Noncentrosymmetric Eu(III) Complexes Are Observably Triboluminescent Not Triboluminescent Na 3[Eu(ODA)3] • 8 H 2 O Eu 2(phen)2(C 8 H 7 O 2)6 ] • 2 H 2 O Cc Cc Z=4 R 1 = 0. 0255 WR 2 = 0. 0699 R 1 = 0. 0547 WR 2 = 0. 1214 Albin, M. ; Whittle, R. R. ; Horrocks, W. D. Inorg. Chem. 1985, 24, 4591. Jin, L. -P. ; Wang, R. -F. Chem. J. Chin. Univ. 1993, 14, 1195.