CENTRIFUGATION THEORY AND PRACTICE Routine centrifuge rotors Calculation


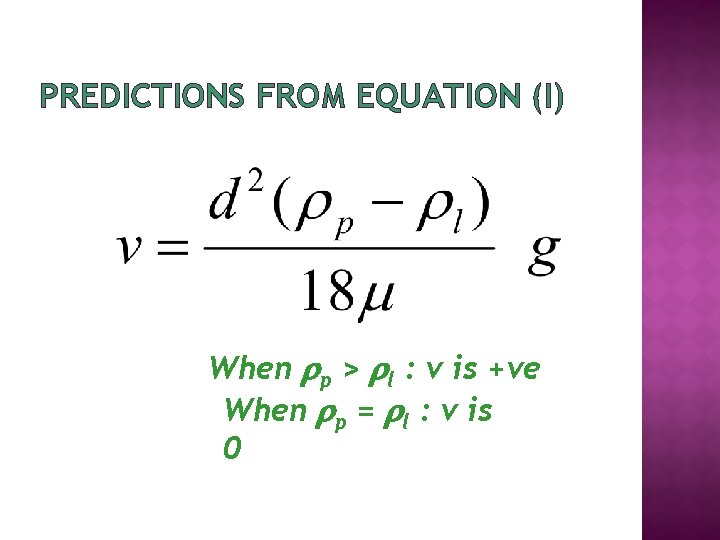







- Slides: 46


CENTRIFUGATION THEORY AND PRACTICE �Routine centrifuge rotors �Calculation of g-force �Differential centrifugation �Density gradient theory

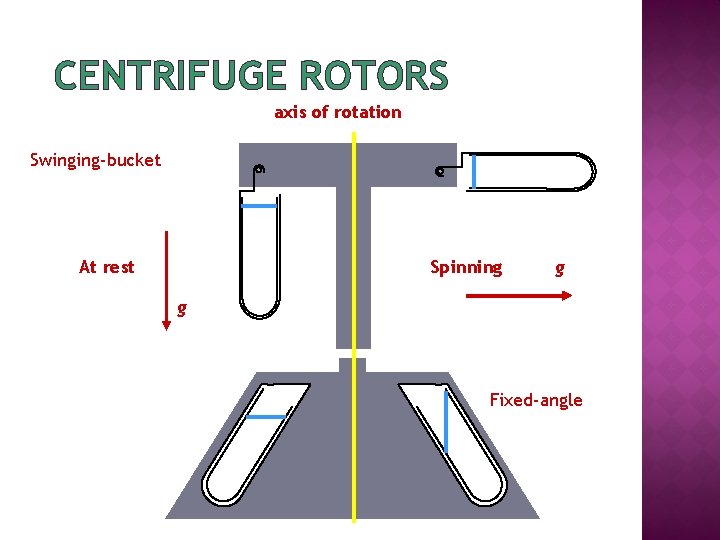
CENTRIFUGE ROTORS axis of rotation Swinging-bucket Spinning At rest g g Fixed-angle

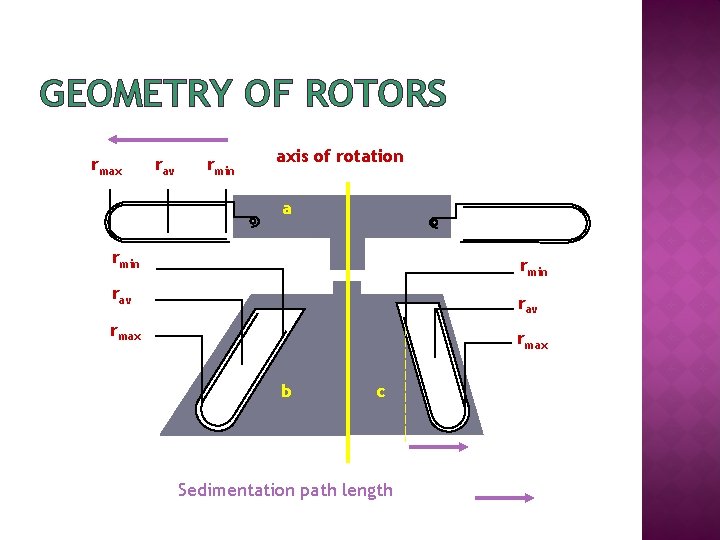
GEOMETRY OF ROTORS rmax rav rmin axis of rotation a rmin rav rmax b c Sedimentation path length

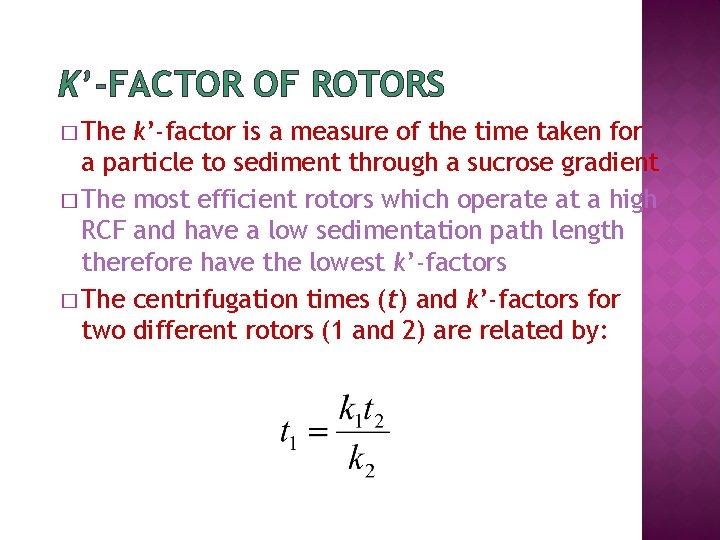
K’-FACTOR OF ROTORS � The k’-factor is a measure of the time taken for a particle to sediment through a sucrose gradient � The most efficient rotors which operate at a high RCF and have a low sedimentation path length therefore have the lowest k’-factors � The centrifugation times (t) and k’-factors for two different rotors (1 and 2) are related by:

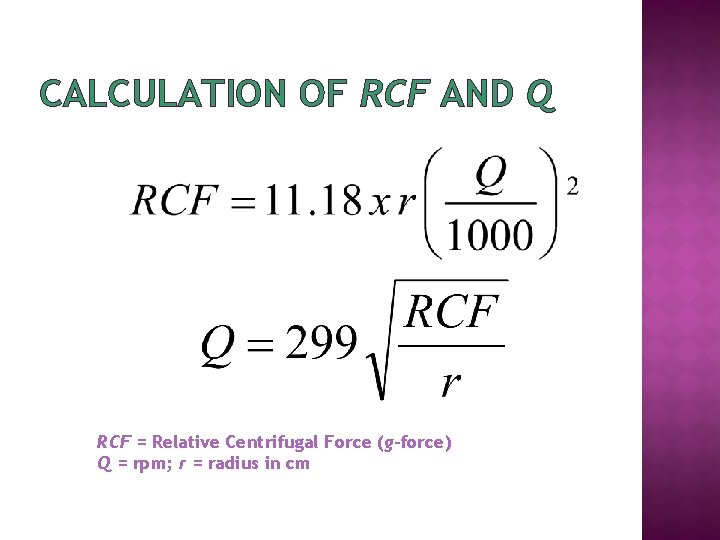
CALCULATION OF RCF AND Q RCF = Relative Centrifugal Force (g-force) Q = rpm; r = radius in cm

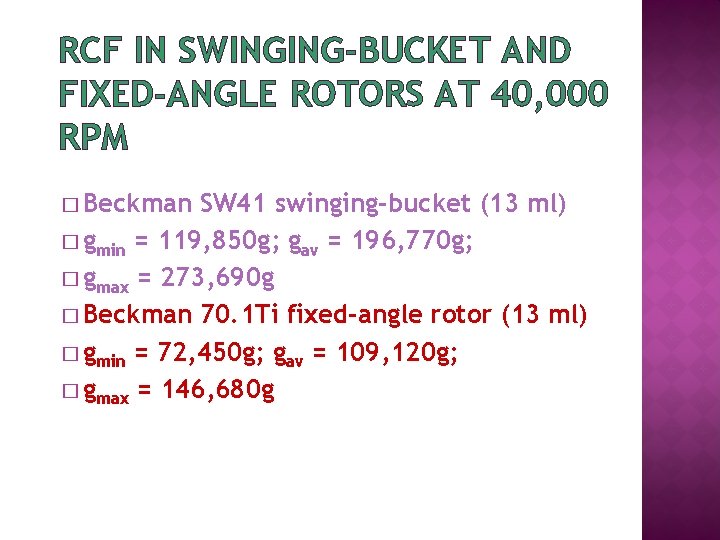
RCF IN SWINGING-BUCKET AND FIXED-ANGLE ROTORS AT 40, 000 RPM � Beckman SW 41 swinging-bucket (13 ml) � gmin = 119, 850 g; gav = 196, 770 g; � gmax = 273, 690 g � Beckman 70. 1 Ti fixed-angle rotor (13 ml) � gmin = 72, 450 g; gav = 109, 120 g; � gmax = 146, 680 g

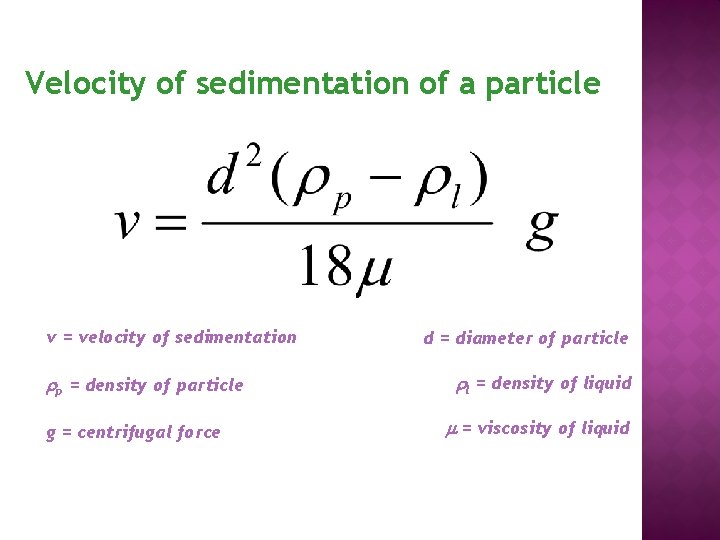
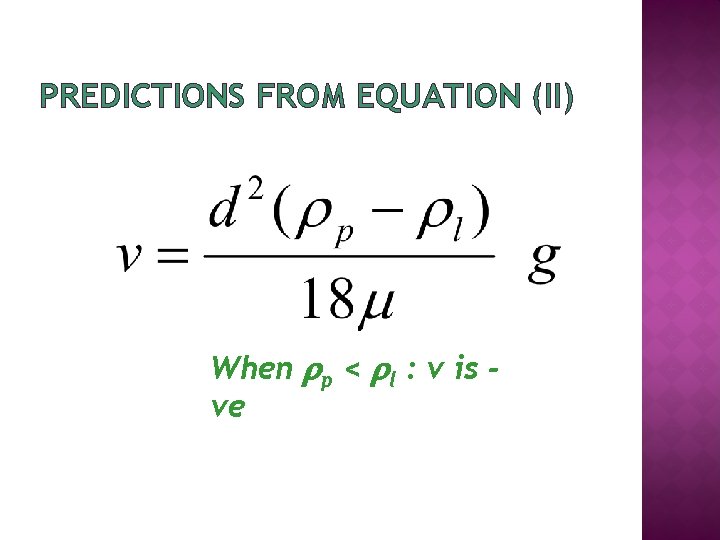
Velocity of sedimentation of a particle v = velocity of sedimentation p = density of particle g = centrifugal force d = diameter of particle l = density of liquid = viscosity of liquid

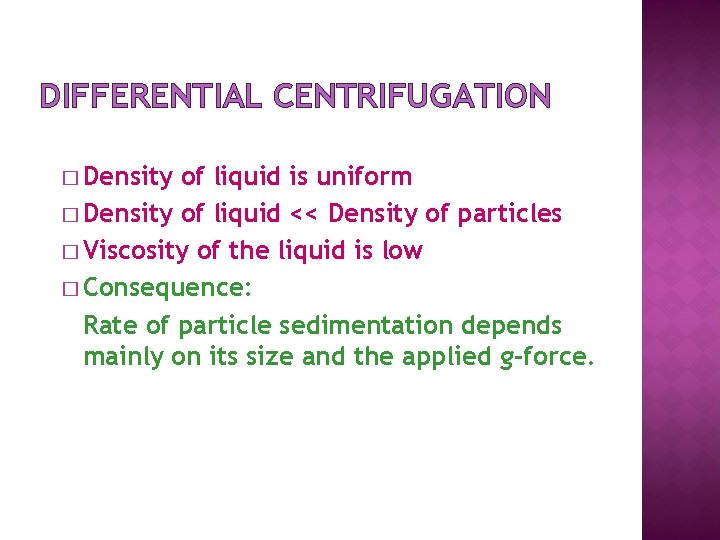
DIFFERENTIAL CENTRIFUGATION � Density of liquid is uniform � Density of liquid << Density of particles � Viscosity of the liquid is low � Consequence: Rate of particle sedimentation depends mainly on its size and the applied g-force.

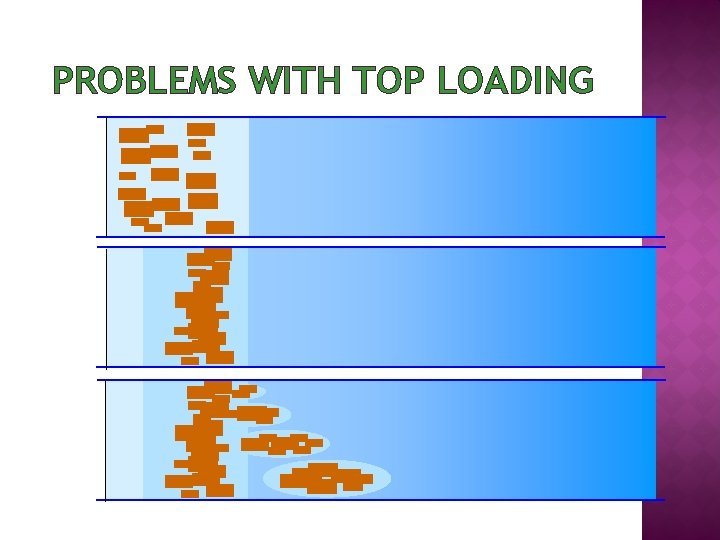
PROBLEMS WITH TOP LOADING

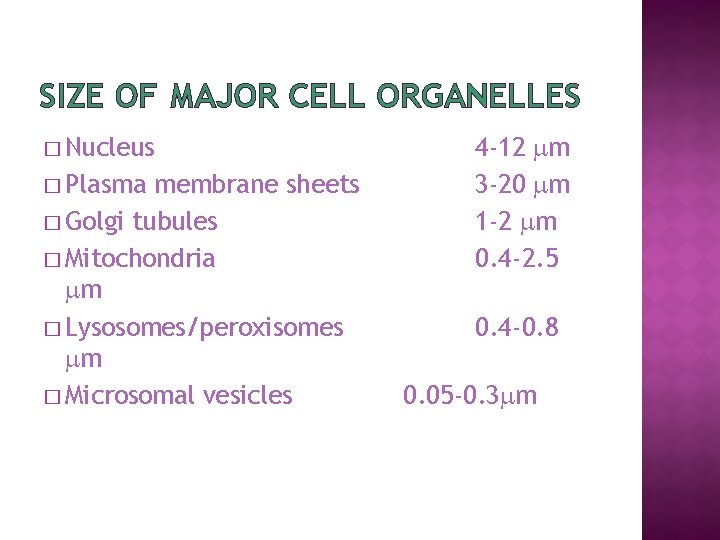
SIZE OF MAJOR CELL ORGANELLES � Nucleus � Plasma membrane sheets � Golgi tubules � Mitochondria m � Lysosomes/peroxisomes m � Microsomal vesicles 4 -12 m 3 -20 m 1 -2 m 0. 4 -2. 5 0. 4 -0. 8 0. 05 -0. 3 m

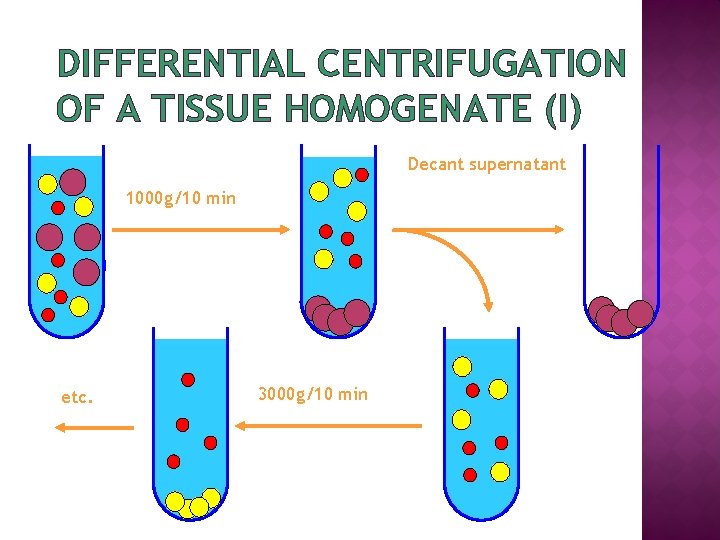
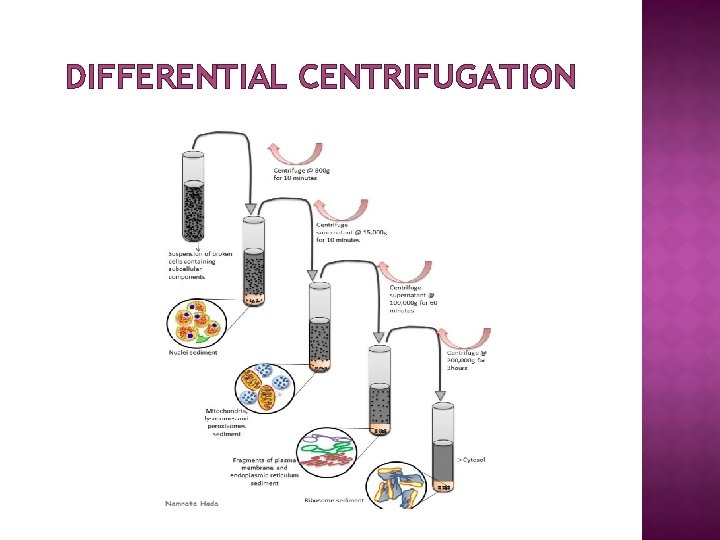
DIFFERENTIAL CENTRIFUGATION OF A TISSUE HOMOGENATE (I) Decant supernatant 1000 g/10 min etc. 3000 g/10 min

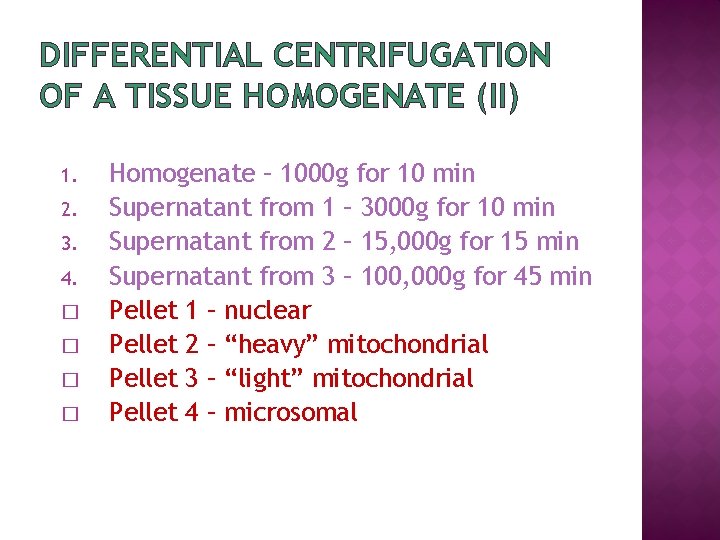
DIFFERENTIAL CENTRIFUGATION OF A TISSUE HOMOGENATE (II) 1. 2. 3. 4. � � Homogenate – 1000 g for 10 min Supernatant from 1 – 3000 g for 10 min Supernatant from 2 – 15, 000 g for 15 min Supernatant from 3 – 100, 000 g for 45 min Pellet 1 – nuclear Pellet 2 – “heavy” mitochondrial Pellet 3 – “light” mitochondrial Pellet 4 – microsomal

DIFFERENTIAL CENTRIFUGATION (III) EXPECTED CONTENT OF PELLETS � 1000 g pellet: nuclei, plasma membrane sheets � 3000 g pellet: large mitochondria, Golgi tubules � 15, 000 g pellet: small mitochondria, lysosomes, peroxisomes � 100, 000 g pellet: microsomes

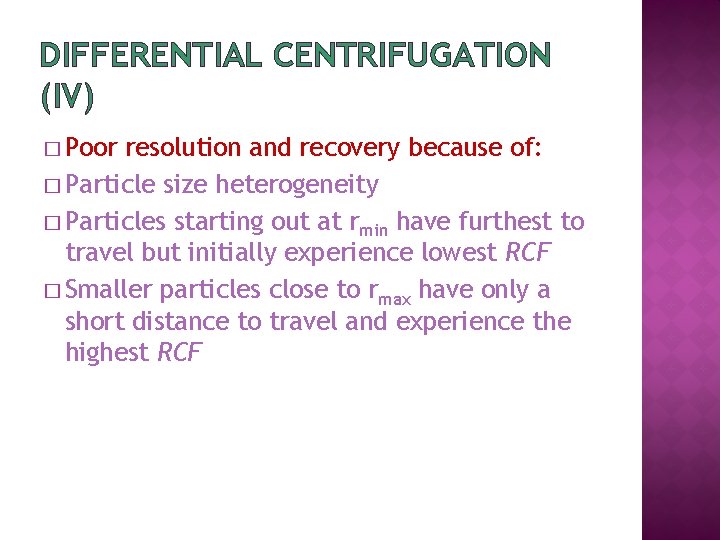
DIFFERENTIAL CENTRIFUGATION (IV) � Poor resolution and recovery because of: � Particle size heterogeneity � Particles starting out at rmin have furthest to travel but initially experience lowest RCF � Smaller particles close to rmax have only a short distance to travel and experience the highest RCF

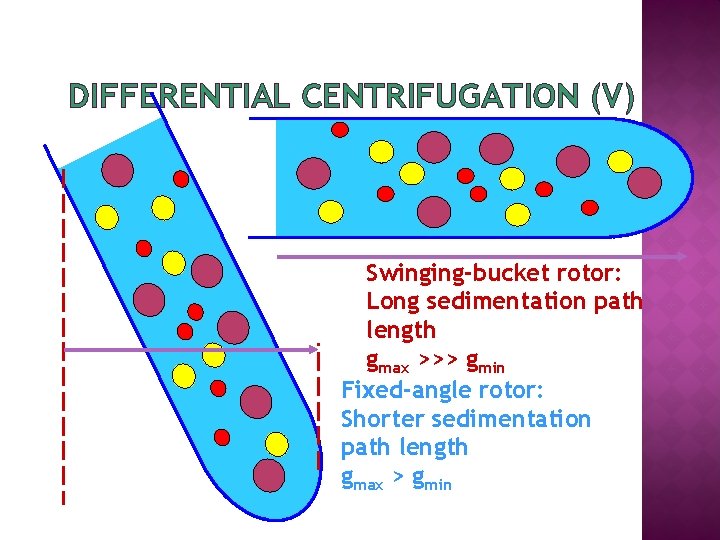
DIFFERENTIAL CENTRIFUGATION (V) Swinging-bucket rotor: Long sedimentation path length gmax >>> gmin Fixed-angle rotor: Shorter sedimentation path length gmax > gmin

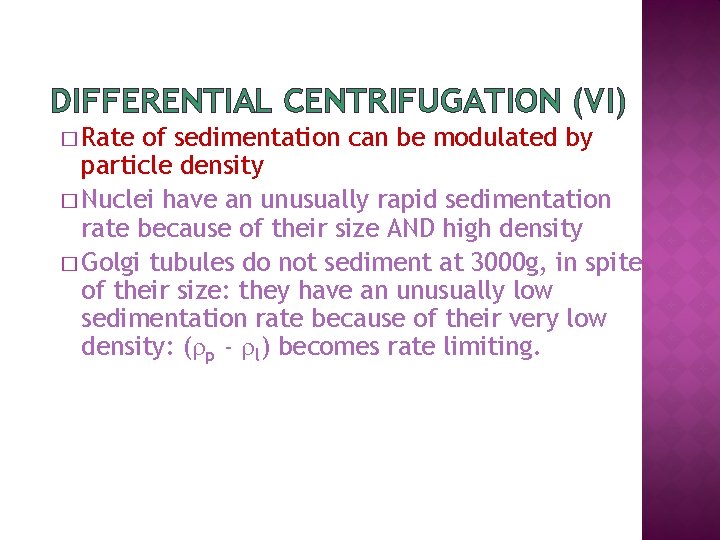
DIFFERENTIAL CENTRIFUGATION (VI) � Rate of sedimentation can be modulated by particle density � Nuclei have an unusually rapid sedimentation rate because of their size AND high density � Golgi tubules do not sediment at 3000 g, in spite of their size: they have an unusually low sedimentation rate because of their very low density: ( p - l) becomes rate limiting.

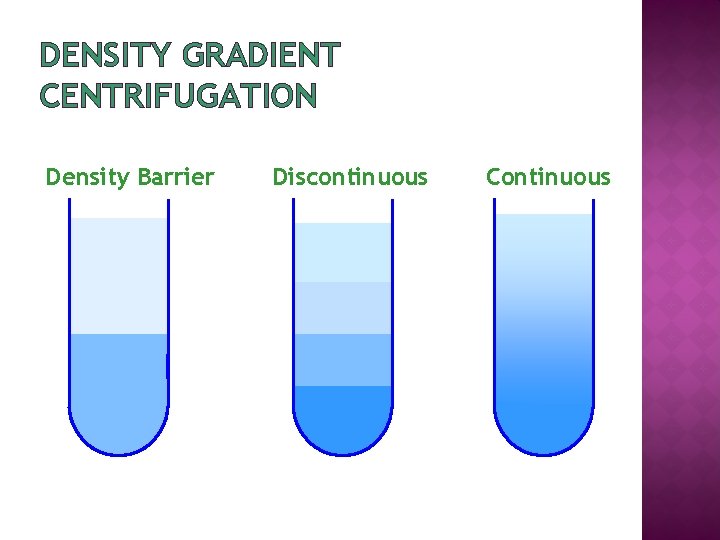
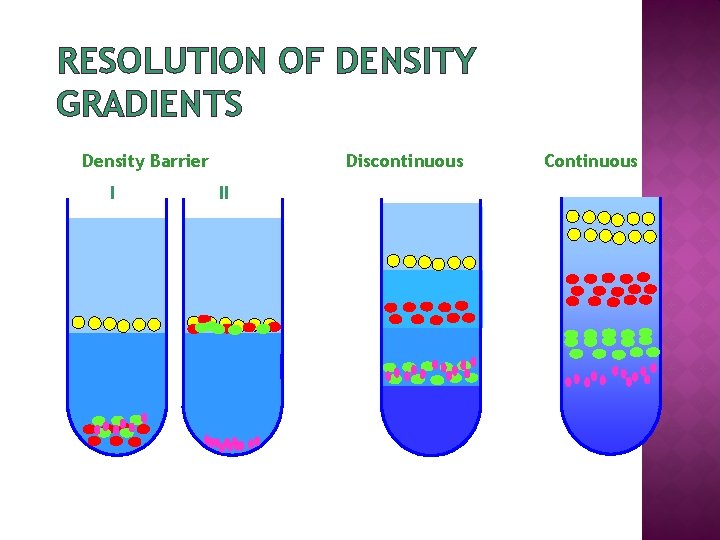
DENSITY GRADIENT CENTRIFUGATION Density Barrier Discontinuous Continuous

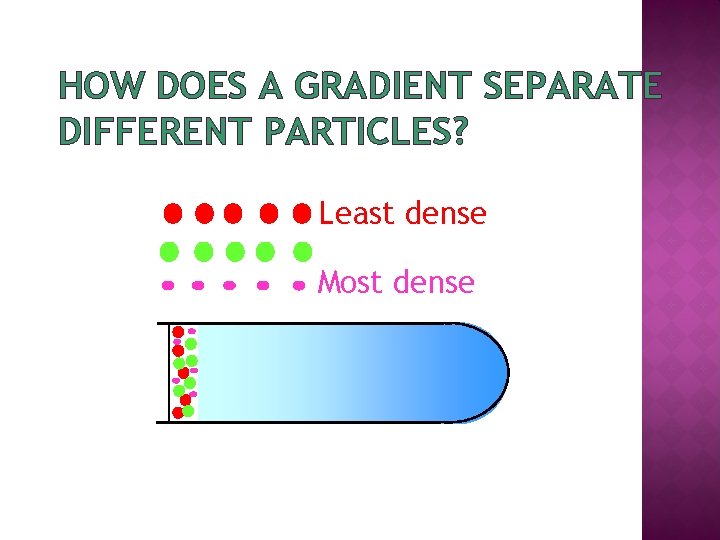
HOW DOES A GRADIENT SEPARATE DIFFERENT PARTICLES? Least dense Most dense
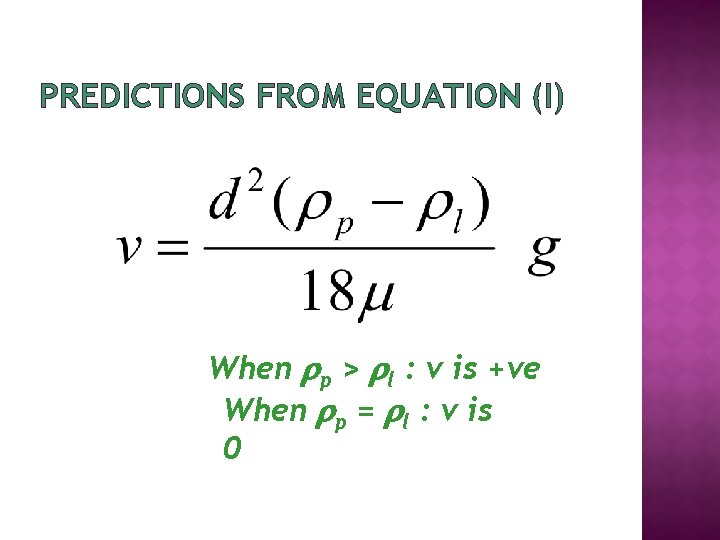
PREDICTIONS FROM EQUATION (I) When p > l : v is +ve When p = l : v is 0

PREDICTIONS FROM EQUATION (II) When p < l : v is ve

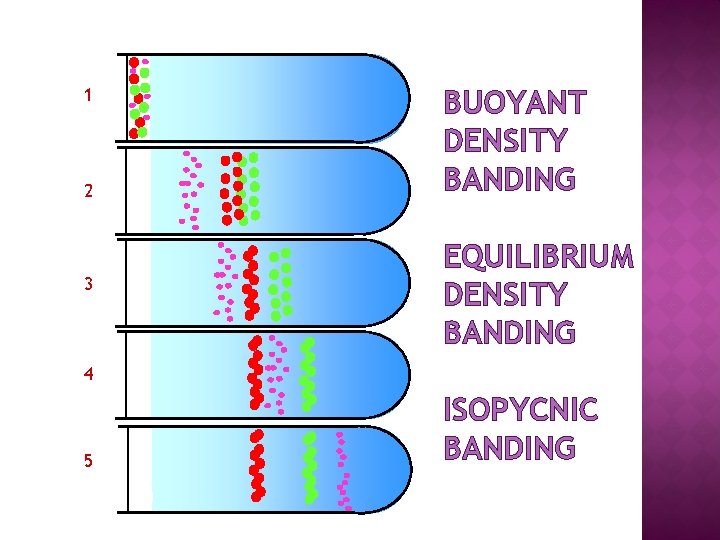
1 2 3 BUOYANT DENSITY BANDING EQUILIBRIUM DENSITY BANDING 4 5 ISOPYCNIC BANDING

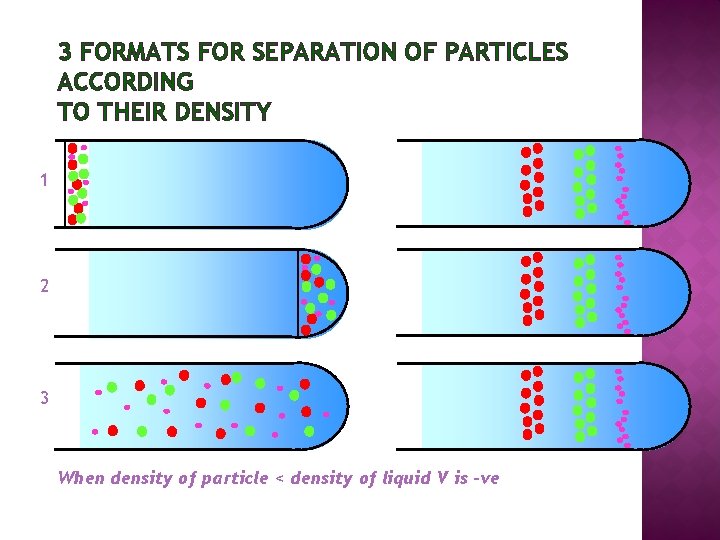
3 FORMATS FOR SEPARATION OF PARTICLES ACCORDING TO THEIR DENSITY 1 2 3 When density of particle < density of liquid V is -ve

RESOLUTION OF DENSITY GRADIENTS Density Barrier I Discontinuous II Continuous

SUMMARY OF PREVIOUS SLIDES �A particle will sediment through a solution if particle density > solution density � If particle density < solution density, particle will float through solution � When particle density = solution density the particle stop sedimenting or floating

PROBLEMS WITH TOP LOADING

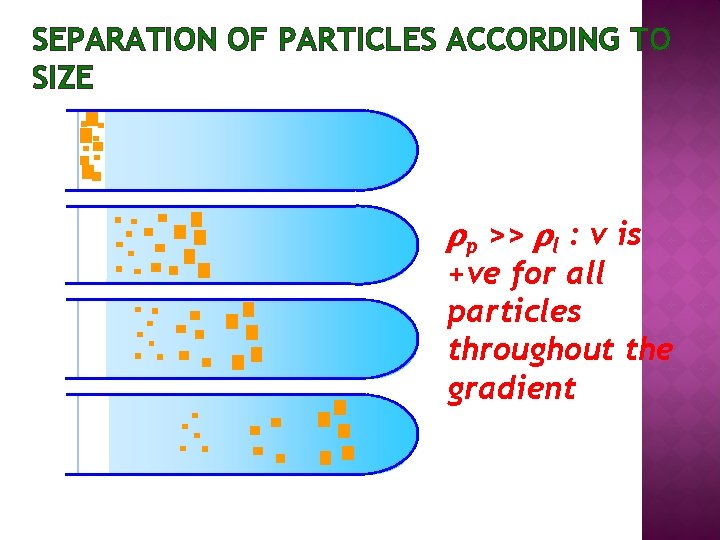
SEPARATION OF PARTICLES ACCORDING TO SIZE p >> l : v is +ve for all particles throughout the gradient

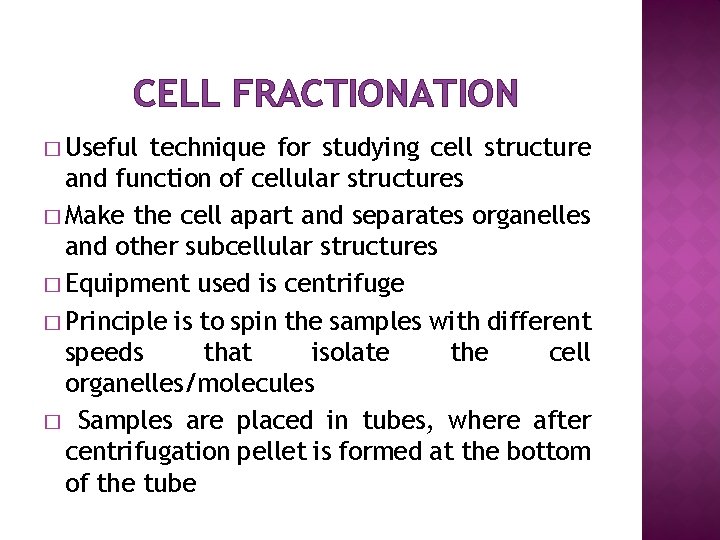
CELL FRACTIONATION � Useful technique for studying cell structure and function of cellular structures � Make the cell apart and separates organelles and other subcellular structures � Equipment used is centrifuge � Principle is to spin the samples with different speeds that isolate the cell organelles/molecules � Samples are placed in tubes, where after centrifugation pellet is formed at the bottom of the tube

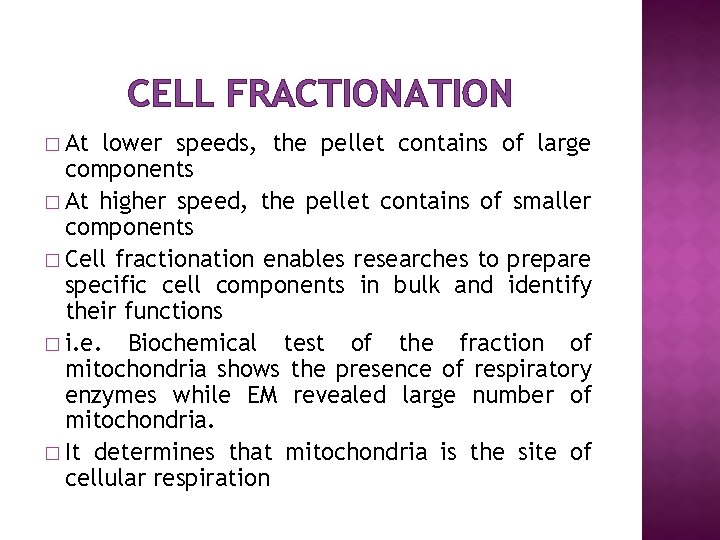
CELL FRACTIONATION � At lower speeds, the pellet contains of large components � At higher speed, the pellet contains of smaller components � Cell fractionation enables researches to prepare specific cell components in bulk and identify their functions � i. e. Biochemical test of the fraction of mitochondria shows the presence of respiratory enzymes while EM revealed large number of mitochondria. � It determines that mitochondria is the site of cellular respiration

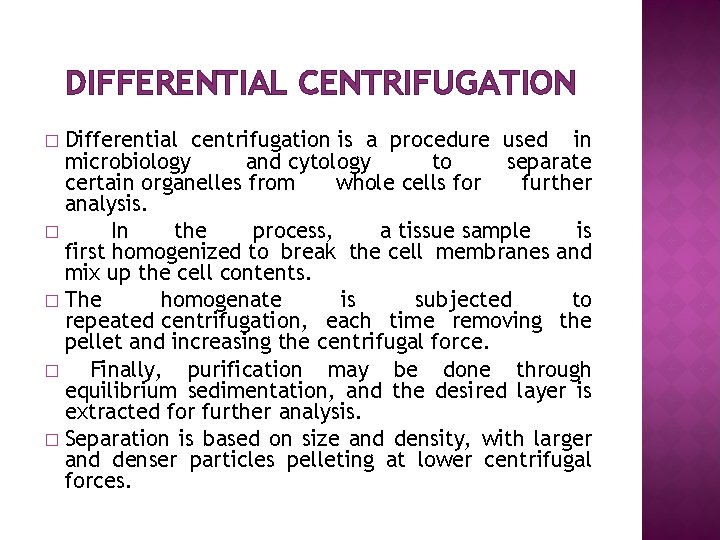
DIFFERENTIAL CENTRIFUGATION Differential centrifugation is a procedure used in microbiology and cytology to separate certain organelles from whole cells for further analysis. � In the process, a tissue sample is first homogenized to break the cell membranes and mix up the cell contents. � The homogenate is subjected to repeated centrifugation, each time removing the pellet and increasing the centrifugal force. � Finally, purification may be done through equilibrium sedimentation, and the desired layer is extracted for further analysis. � Separation is based on size and density, with larger and denser particles pelleting at lower centrifugal forces. �

DIFFERENTIAL CENTRIFUGATION

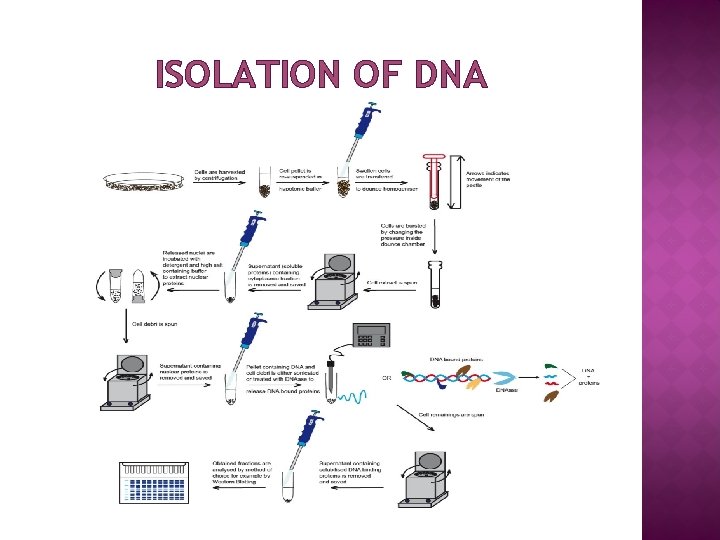
ISOLATION OF DNA

SEPARATION OF LIQUIDS

TABLE TOP MICROCENTRIFUGE (SIGMA)

TABLE TOP CENTRIFUGES

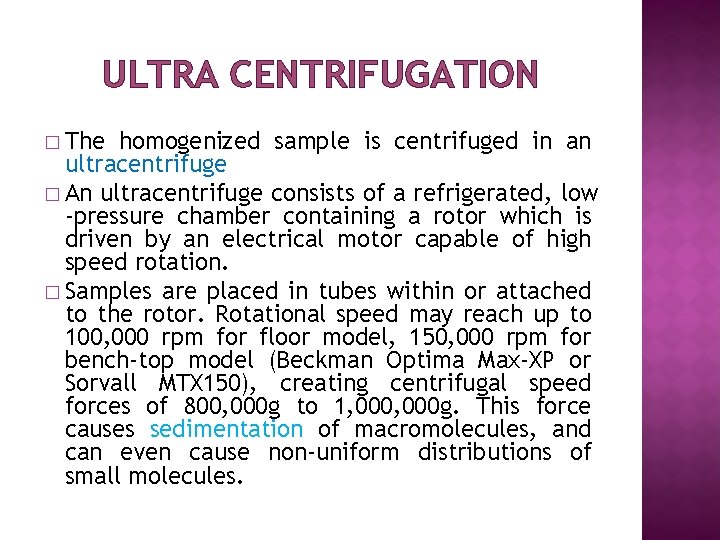
ULTRA CENTRIFUGATION � The homogenized sample is centrifuged in an ultracentrifuge � An ultracentrifuge consists of a refrigerated, low -pressure chamber containing a rotor which is driven by an electrical motor capable of high speed rotation. � Samples are placed in tubes within or attached to the rotor. Rotational speed may reach up to 100, 000 rpm for floor model, 150, 000 rpm for bench-top model (Beckman Optima Max-XP or Sorvall MTX 150), creating centrifugal speed forces of 800, 000 g to 1, 000 g. This force causes sedimentation of macromolecules, and can even cause non-uniform distributions of small molecules.

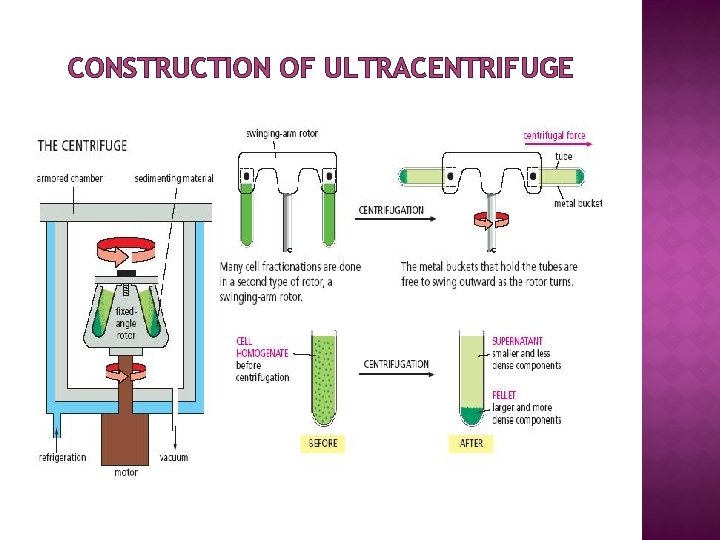
CONSTRUCTION OF ULTRACENTRIFUGE

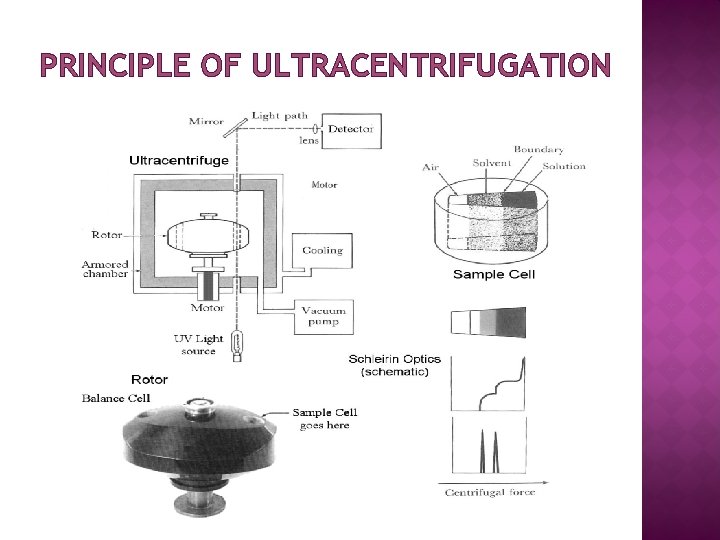
PRINCIPLE OF ULTRACENTRIFUGATION

TABLE TOP ULTRA CENTRIFUGE

ULTRACENTRIFUGE

ROTORS OF ULTRACENTRIFUGE

ROTERS OF ULTRACENTRIFUGE

DAMAGE OF ULTRACENTRIFUGE

ISOPYCNIC CENTRIFUGATION � Isopycnic centrifugation (density gradient centrifugation or equilibrium sedimentation) separates molecules on the basis of buoyant density. Typically, a "selfgenerating" density gradient is established via equilibrium sedimentation, and then analyte molecules concentrate as bands where the molecule density matches that of the surrounding solution. � For fractionation of nucleic acids, the mixture of Caesium chloride and DNA is centrifuged for several hours at high speed. Caesium chloride concentration of 1. 6 to 1. 8 g/m. L is similar to the density of DNA. � A gradient of Caesium ions is formed, due to two opposing forces: diffusion and centrifugal force. The caesium ions will sediment away from the rotor, and become more concentrated near the bottom of the tube. The diffusive force arises due to the concentration gradient of solvated caesium chloride and is always directed towards the center of the rotor. The balance between these two forces generates a stable density gradient in the caesium chloride solution, which is more dense near the bottom of the tube, and less dense near the top.

ISOPYCNIC CENTRIFUGATION � The DNA molecules will be separated based on the relative proportions of AT (adenine and thymine base pairs) to GC (guanine and cytosine base pairs). � An AT base pair has a lower molecular weight than a GC base pair and thus, for two DNA molecules of equal length, the one with the greater proportion of AT base pairs will have a lower density � Different types of nucleic acids will also be separated into bands, e. g. RNA is denser than super coiled plasmid DNA, which is denser than linear chromosomal DNA.

SUCROSE GRADIENT CENTRIFUGATION � Sucrose gradient centrifugation is used to purify enveloped viruses (with densities 1. 1 -1. 2 g/cm³), ribosome, membranes and so on. � This method is also used to purify exosomes. � There are two methods - equilibrium centrifugation and non-equilibrium centrifugation. � Typically in equilibrium centrifugation, a sucrose density gradient is created by gently overlaying lower concentrations of sucrose on higher concentrations in a centrifuge tube. For example, a sucrose gradient may consist of layers extending from 70% sucrose to 20% sucrose in 10% increments