Centre for Geothermal Research 1 Centre de Gochimie








- Slides: 18

Centre for Geothermal Research (1) Centre de Géochimie de la Surface (2) Neuchâtel - Switzerland Strasbourg - France Overview of chemical stimulations for EGS and non EGS reservoirs François-D. VUATAZ 1, Bertrand FRITZ 2 and Laurent ANDRE 1 ENGINE Launching Conference BRGM - Orléans, February 14 th, 2006

Plan of the presentation Ø Acid treatments of reservoirs Ø Methodology Ø Different types of acidizing processes Ø Chemical compounds Ø Short inventory of reactive agents Ø Mostly used compounds and their properties Examples of geothermal reservoir acidification Ø Acidification of high temperature geothermal wells Ø Chemical stimulation of EGS reservoirs Ø The case of Soultz

Acid treatment of reservoirs Aims Enhancement of well productivity; Reduction of skin factor by removing near-wellbore damage; Dissolution of scaling deposits in fractures. Technology mainly developed and applied for the development of oil reservoirs. Technology frequently applied for the cleaning and the stimulation of high temperature geothermal reservoirs. Acidizing operation, 1932

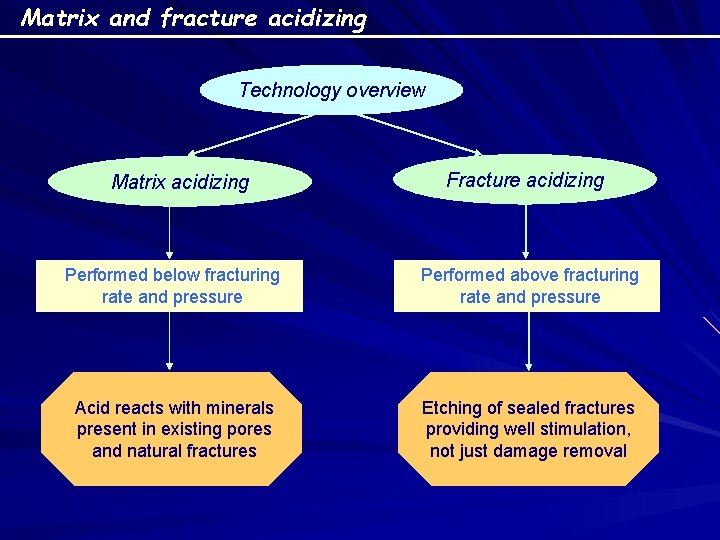
Matrix and fracture acidizing Technology overview Matrix acidizing Fracture acidizing Performed below fracturing rate and pressure Performed above fracturing rate and pressure Acid reacts with minerals present in existing pores and natural fractures Etching of sealed fractures providing well stimulation, not just damage removal

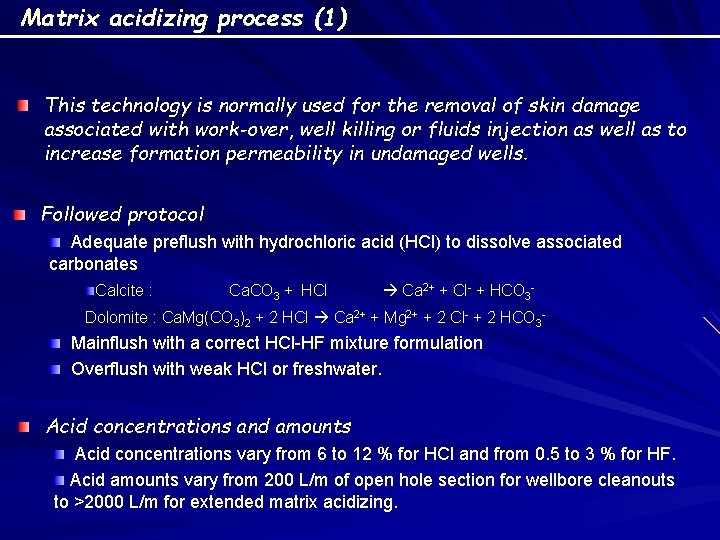
Matrix acidizing process (1) This technology is normally used for the removal of skin damage associated with work-over, well killing or fluids injection as well as to increase formation permeability in undamaged wells. Followed protocol Adequate preflush with hydrochloric acid (HCl) to dissolve associated carbonates Calcite : Ca. CO 3 + HCl Ca 2+ + Cl- + HCO 3 - Dolomite : Ca. Mg(CO 3)2 + 2 HCl Ca 2+ + Mg 2+ + 2 Cl- + 2 HCO 3 - Mainflush with a correct HCl-HF mixture formulation Overflush with weak HCl or freshwater. Acid concentrations and amounts Acid concentrations vary from 6 to 12 % for HCl and from 0. 5 to 3 % for HF. Acid amounts vary from 200 L/m of open hole section for wellbore cleanouts to >2000 L/m for extended matrix acidizing.

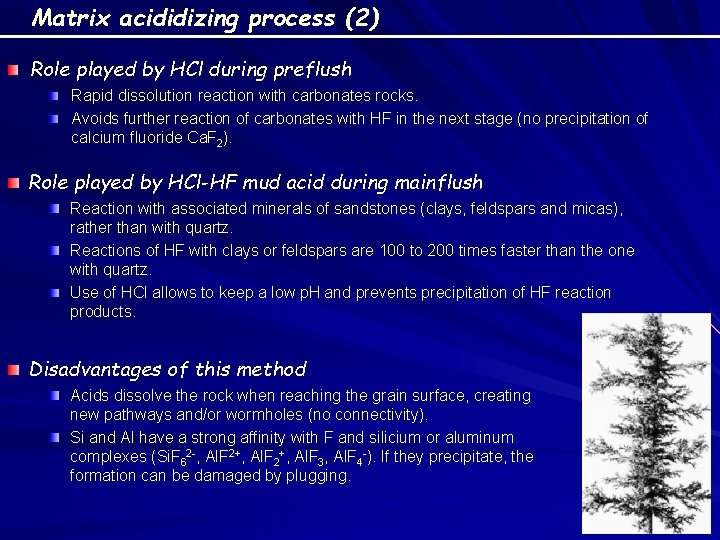
Matrix acididizing process (2) Role played by HCl during preflush Rapid dissolution reaction with carbonates rocks. Avoids further reaction of carbonates with HF in the next stage (no precipitation of calcium fluoride Ca. F 2). Role played by HCl-HF mud acid during mainflush Reaction with associated minerals of sandstones (clays, feldspars and micas), rather than with quartz. Reactions of HF with clays or feldspars are 100 to 200 times faster than the one with quartz. Use of HCl allows to keep a low p. H and prevents precipitation of HF reaction products. Disadvantages of this method Acids dissolve the rock when reaching the grain surface, creating new pathways and/or wormholes (no connectivity). Si and Al have a strong affinity with F and silicium or aluminum complexes (Si. F 62 -, Al. F 2+, Al. F 3, Al. F 4 -). If they precipitate, the formation can be damaged by plugging.

Fracture acidizing Also called acid fraccing, two main techniques could be used: The fluid-loss control contains the acid in natural or newly opened fractures (use of packers). A viscous fluid is injected at a rate higher than the reservoir matrix will accept leading to cracking of the rock. Continued fluid injection increases the fracture’s length and width and injected HCl acid reacts all along the fracture to create a flow channel that extends deep into the formation. The key to success is the penetration of reactive acid along the fracture. The treatment volumes for fracture acidizing are much larger than for matrix acidizing treatment, being as high as 12 000 25 000 L/m of open hole.

Chemical compounds Reactive agents for carbonates and silicates Hydrochloric acid (HCl) and hydrochloric-hydrofluoric (HCl-HF) mud acid Acetic acid (CH 3 COOH) and chloroacetic acid (Cl. CH 2 COOH) Formic acid (HCOOH) Sulfamic acid (H 2 NSO 3 H) Chelatants (EDTA) Reactive agents for quartz Sodium carbonate (Na 2 CO 3). Additives Corrosion inhibitor to protect casings Anti-sludge agents Iron chelating agents Retardants to prolong the effect of the reactive agent further in the fractures Different solvents according to the treated formation.

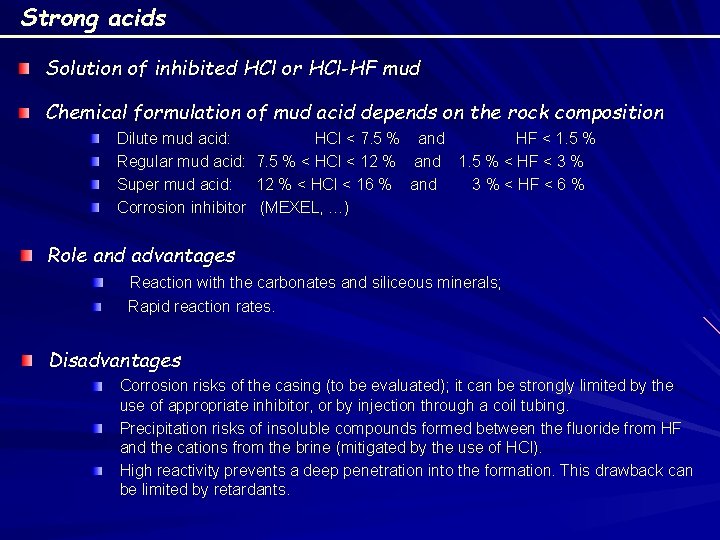
Strong acids Solution of inhibited HCl or HCl-HF mud Chemical formulation of mud acid depends on the rock composition Dilute mud acid: HCl < 7. 5 % and HF < 1. 5 % Regular mud acid: 7. 5 % < HCl < 12 % and 1. 5 % < HF < 3 % Super mud acid: 12 % < HCl < 16 % and 3 % < HF < 6 % Corrosion inhibitor (MEXEL, …) Role and advantages Reaction with the carbonates and siliceous minerals; Rapid reaction rates. Disadvantages Corrosion risks of the casing (to be evaluated); it can be strongly limited by the use of appropriate inhibitor, or by injection through a coil tubing. Precipitation risks of insoluble compounds formed between the fluoride from HF and the cations from the brine (mitigated by the use of HCl). High reactivity prevents a deep penetration into the formation. This drawback can be limited by retardants.

Weak acids Mixture containing organic acid and HF Chemical formulation 9 % formic acid (or 10 % acetic acid) Corrosion inhibitor (MEXEL, …) Role and advantages Reaction with the carbonates and siliceous minerals; Dissolving capacity 25 % higher than HCl; p. H higher than for strong acids, limiting the corrosion risks and the amounts of corrosion inhibitor; Reaction rate slower than for HCl, allowing a better penetration into the formation. Disadvantages Corrosion risks on the casing (to be evaluated); risks can be strongly limited by the use of appropriate inhibitor, or by injection through a coil tubing.

Chelating agents These solutions are used as formation cleanup and for stimulating oil and gas wells; especially in formations that may be damaged by strong acids. Chemical formulation EDTA (Ethylenediaminetetraacetic acid), HEDTA (Hydroxyethylenediaminetriacetic acid), HEIDA (Hydroxyethyliminodiacetic acid) HCl Corrosion inhibitor (MEXEL, …) Role and advantages Acting as a solvent, increasing the water-wetting properties and dissolving (entirely or partially) minerals containing Fe, Ca, Mg and Al; Dissolving capacity 50 % higher than HCl; p. H higher than HCl, limiting corrosion risks and amounts of corrosion inhibitor; Reaction rate slower than for HCl allowing a better penetration into the formation. Disadvantages Corrosion risks are more limited than with strong or weak acids. Environmental problems in case of fluid discharge.

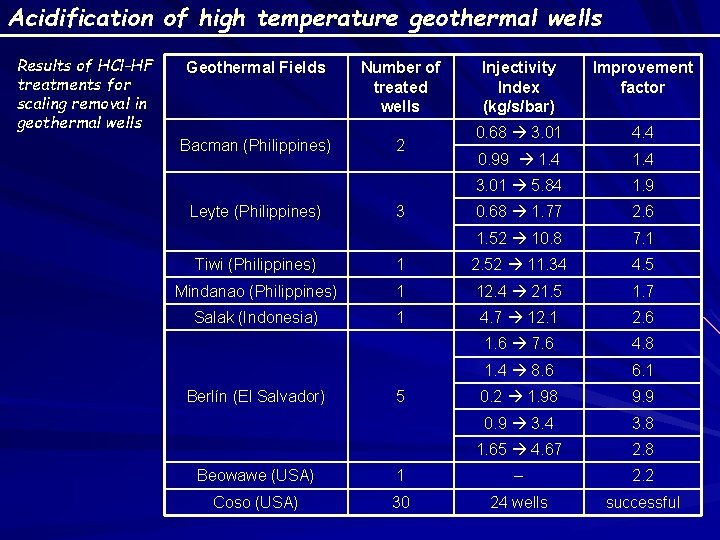
Acidification of high temperature geothermal wells Results of HCl-HF treatments for scaling removal in geothermal wells Geothermal Fields Number of treated wells Bacman (Philippines) 2 Leyte (Philippines) 3 Injectivity Index (kg/s/bar) Improvement factor 0. 68 3. 01 4. 4 0. 99 1. 4 3. 01 5. 84 1. 9 0. 68 1. 77 2. 6 1. 52 10. 8 7. 1 Tiwi (Philippines) 1 2. 52 11. 34 4. 5 Mindanao (Philippines) 1 12. 4 21. 5 1. 7 Salak (Indonesia) 1 4. 7 12. 1 2. 6 1. 6 7. 6 4. 8 1. 4 8. 6 6. 1 0. 2 1. 98 9. 9 0. 9 3. 4 3. 8 1. 65 4. 67 2. 8 Berlín (El Salvador) 5 Beowawe (USA) 1 – 2. 2 Coso (USA) 30 24 wells successful

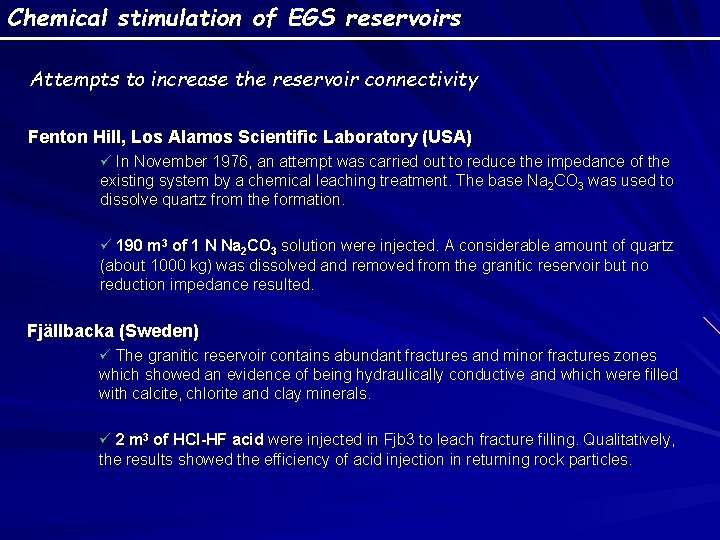
Chemical stimulation of EGS reservoirs Attempts to increase the reservoir connectivity Fenton Hill, Los Alamos Scientific Laboratory (USA) ü In November 1976, an attempt was carried out to reduce the impedance of the existing system by a chemical leaching treatment. The base Na 2 CO 3 was used to dissolve quartz from the formation. ü 190 m 3 of 1 N Na 2 CO 3 solution were injected. A considerable amount of quartz (about 1000 kg) was dissolved and removed from the granitic reservoir but no reduction impedance resulted. Fjällbacka (Sweden) ü The granitic reservoir contains abundant fractures and minor fractures zones which showed an evidence of being hydraulically conductive and which were filled with calcite, chlorite and clay minerals. ü 2 m 3 of HCl-HF acid were injected in Fjb 3 to leach fracture filling. Qualitatively, the results showed the efficiency of acid injection in returning rock particles.

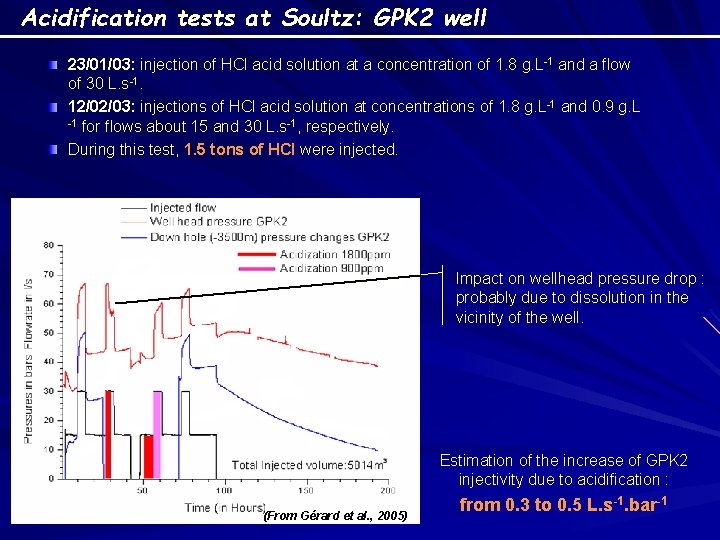
Acidification tests at Soultz: GPK 2 well 23/01/03: injection of HCl acid solution at a concentration of 1. 8 g. L-1 and a flow of 30 L. s-1. 12/02/03: injections of HCl acid solution at concentrations of 1. 8 g. L-1 and 0. 9 g. L -1 for flows about 15 and 30 L. s-1, respectively. During this test, 1. 5 tons of HCl were injected. Impact on wellhead pressure drop : probably due to dissolution in the vicinity of the well. Estimation of the increase of GPK 2 injectivity due to acidification : (From Gérard et al. , 2005) from 0. 3 to 0. 5 L. s-1. bar-1

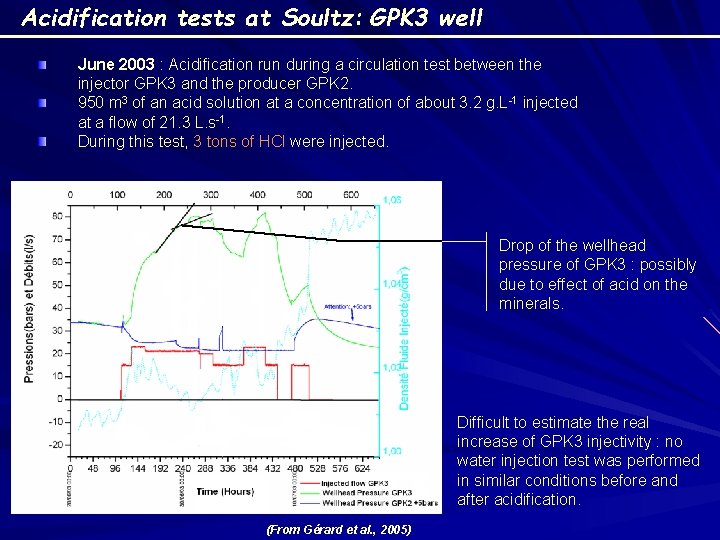
Acidification tests at Soultz: GPK 3 well June 2003 : Acidification run during a circulation test between the injector GPK 3 and the producer GPK 2. 950 m 3 of an acid solution at a concentration of about 3. 2 g. L-1 injected at a flow of 21. 3 L. s-1. During this test, 3 tons of HCl were injected. Drop of the wellhead pressure of GPK 3 : possibly due to effect of acid on the minerals. Difficult to estimate the real increase of GPK 3 injectivity : no water injection test was performed in similar conditions before and after acidification. (From Gérard et al. , 2005)

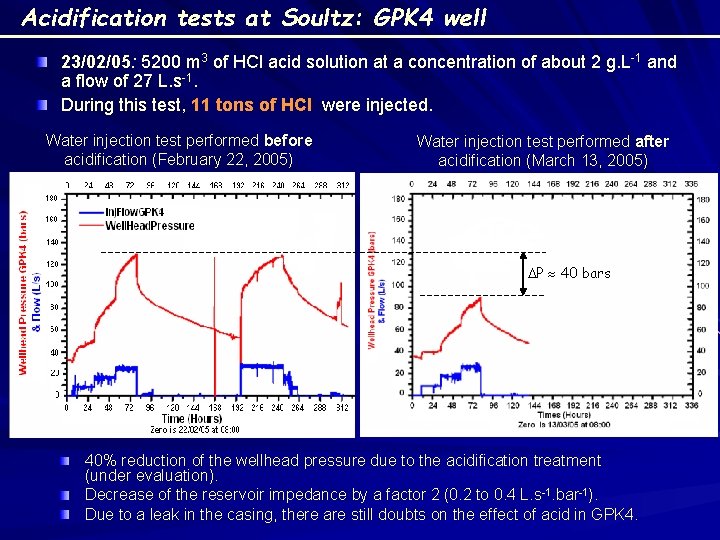
Acidification tests at Soultz: GPK 4 well 23/02/05: 5200 m 3 of HCl acid solution at a concentration of about 2 g. L-1 and a flow of 27 L. s-1. During this test, 11 tons of HCl were injected. Water injection test performed before acidification (February 22, 2005) Water injection test performed after acidification (March 13, 2005) DP 40 bars 40% reduction of the wellhead pressure due to the acidification treatment (under evaluation). Decrease of the reservoir impedance by a factor 2 (0. 2 to 0. 4 L. s-1. bar-1). Due to a leak in the casing, there are still doubts on the effect of acid in GPK 4.

Preliminary conclusions on the chemical stimulation Long and successful experience acquired from the oil industry ü Large number of methods and experiences set up for oil and gas wells. ü Effect of acid stimulation is usually limited to the first metres around the wells. üProcedures are partially adapted to geothermal reservoirs. High temperature geothermal fields ü Numerous wells in geothermal fields have been chemically stimulated, mostly by strong acids (Philippines, El Salvador, USA, Italy). ü Mineral deposits on casings and around the well are treated successfully several times per year at Heber geothermal field, California. ü Corrosion damage can be mostly avoided by using adequate inhibitors. EGS reservoirs ü Old projects: only a few chemical stimulations were realised (Fenton Hill, Fjällbacka). ü The Soultz EGS has probably the best experience on soft HCl stimulation so far. ü Modelling the effect of acid stimulation for the Soultz reservoir is under progress. ü New experiments are planned to stimulate GPK 4 well and to connect it to major fractures.
