CENTRAL STERILIZATION AND SUPPLIES DIVISION Infection Control Nursing






































- Slides: 44

CENTRAL STERILIZATION AND SUPPLIES DIVISION Infection Control Nursing Officer Infection Control Unit Teaching Hospital Jaffna. 1

Definition of terms Sterilization – complete elimination or destruction of all forms of microbial life (including spores) Disinfection – elimination of micro-organisms on inanimate objects with the exception of bacterial spores Antisepsis – prevention of infection of tissues and body surfaces by application of germicides Cleaning – the removal of visible soil (eg; inorganic and organic material) from objects and surfaces 2

Disinfection and sterilization practices are essential for ensuring that medical and surgical instruments do not transmit infectious pathogens to patients 3

Instruments and items for patient care divided into 3 categories based on the degree of risk of infection involved in the use of the items 1. Critical/ high risk items 2. Semicritical/ intermediate risk items 3. Non critical/ low risk items 4

1. Critical / high risk items Objects that enter sterile body tissue or vascular system Any microbial contamination could result in disease transmission Must be sterile 5

2. Semi-critical / intermediate risk items Items that come in contact with intact mucous membranes Generally resistant to infection by common bacterial spores but susceptible to other micro-organisms Need not be sterile; disinfection acceptable 6

3. Non-critical / low risk items Items that come into contact with intact skin but not mucous membranes Intact skin is an effective barrier to most microorganisms, hence low risk of disease transmission Thorough cleaning is adequate with low level disinfection as necessary 7

Factors influence the sterilization process The temperature of sterilization The period of sterilization Inactivation by organic matter Whether the article was cleaned before sterilization The sterilization cycle can be divided into three periods: Ø a. Heating up period Ø b. Holding period Ø c. Cooling period 8

CENTRAL STERILE SUPPLY DIVISION

Objective To reduce hospital associated infections and protect patients, employees, health care students and visitors.

The functional flow of activities in CSSD RINSING CLEANING DRYING INSPECTION AND ASSEMBLY PACKAGING LABELLING STERILIZATION STORAGE DISTRIBUTION

RINSING Rinsing of articles after use should not be permitted in patient care areas unless carried out by a trained member of the staff.

CLEANING All reusable medical devices should be thoroughly cleaned prior to disinfection or sterilization.

DRYING All articles should be dried appropriately

INSPECTION AND ASSEMBLY Each item should be inspected for functionality, defects, breakage and then appropriately assembled.

PACKAGING Articles should preferably be packed in porous material.

LABELLING Each pack should be marked with nomenclature of the article, contents of the pack, initials of the person who packed it, date and initials of the person who carried out the sterilization.

STORAGE Should be properly managed separately for sterile and non-sterile stores. For sterile goods, clean room conditions should be followed. Sterile pack should be stored 20 -25 cm from the floor, 45 -50 cm from the ceiling, 15 -20 cm on out side of the wall.

DISTRIBUTION Refers to clean and dirty articles exchange system. A program should be established for the collection of used items from patient care areas and distribution of sterilized goods.

Moist heat sterilization 1. 2. 3. 4. Moist heat kills microorganisms by coagulating and denaturing enzymes and structural proteins Applied in the form of steam Steam under pressure is used to achieve a higher temperature 4 parameters of steam sterilization Steam – saturated and dry steam without air Pressure – 2. 4 bar / 15 lb Temperature – 1210 C Time - 20 min Autoclaving at 1210 C at 15 lb pressure for 20 min is acceptable for routine hospital use 20

Moist heat sterilization… Advantages ü Non toxic ü Inexpensive ü Rapid action ü Good penetration ability 21

Sterilization by dry heat Dry heat penetrates less well and is less effective than moist heat Consequently, higher temperatures and longer periods are required for sterilization with dry heat Used for sterilization of -sharp cutting instruments and -laboratory glass wear -powder, oil 22

Sterilization by dry heat Total cycle may be several hours, hence hot air ovens should have Ø Time lock on the door - items cannot be added or removed during, the cycle Ø Fan to distribute the heat evenly Dry heating at 1600 C for 2 hours is acceptable for routine hospital use 23

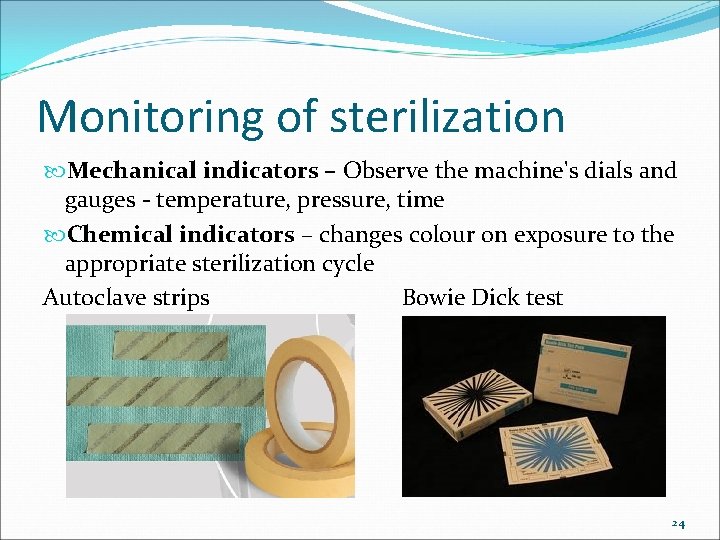
Monitoring of sterilization Mechanical indicators – Observe the machine's dials and gauges - temperature, pressure, time Chemical indicators – changes colour on exposure to the appropriate sterilization cycle Autoclave strips Bowie Dick test 24

Monitoring of sterilization… Biological indicators – bacterial spores which require high temperatures to lose viability – spore strips, self contained vials ØGeobacillus stearothermophilus – for steam or chemical vapor sterilization ØBacillus subtilis -for dry-heat or ethylene oxide gas sterilization 25

26

Storage Once sterilized, the instruments should be maintained in a sterile state until they are used again Improper storage would result in a break of the 'chain of Sterility' The expected 'shelf-life' of a sterile package is depend on the type of area in which it is stored and the material used for packaging A closed, protected area with a minimal airflow such as a cabinet or drawer that can easily be disinfected is preferable to an open stacking system 27

Disinfection Methods Heat – Ø Pasteurization - 63 -66 0 C for 30 min 72 0 C for 15 sec. (the flash method) Ø Boiling - bacterial spores can survive Physical – ultrasonic, UV radiation - used for removing debris (cleaning) prior to autoclaving Chemical Ø Disinfectant used for one purpose may not be equally effective for another Ø The antimicrobial activity falls in the presence of organic debris 28

Disinfection & Sterilization 2% Glutaraldehyde (cidex) - 2% Alkaline solution - Needs activation - Sterilization time – 10 hours - Disinfection time – 20 mins - Activated solution – 2 weeks/20 times - Sterilization/HLD of heat sensitive items - Irritant to skin, eyes & respiratory tract - Should be handled in a well ventilated area - Should wear protective clothing - Fixation of protein

Disinfection & Sterilization (cont. ) Para Safe - Combination of per oxygen compound, activator & stabilizer - Non-corrosive - Non-irritant to skin - Eye irritation - No toxic residues - Expensive - Stable for 24 hours only

Disinfection & Sterilization (cont. ) Chlorine releasing agents - Sodium hypochlorite, Calcium hypochlorite, Chlorine granules - Active against bacteria, viruses, fungi & some spores - Less active against mycobacteria - Contact time 20 -30 mins - Rapidly inactivated by organic matter - Corrosive to metal - Do not add powder to water - Avoid contact with acid/acidic body fluids e. g. - urine - Avoid contact with skin, eyes & mucus membrane - Unstable - daily preparation is necessary - Blood & body fluid spillages – 1% solution (10000 ppm) - General environmental cleaning – 0. 1% (1000 ppm)

Disinfection & Sterilization (cont. ) Alcohol - 70% - 90% Ethyl alcohol/ Isopropyl alcohol - Rapidly active against vegetative bacteria & mycobacteria - Viruses – variable activity - Not sporicidal - Only on clean surfaces - Disinfection of items that cannot be immersed in other disinfectants e. g. - electric equipment - Skin anti-sepsis

Disinfection & Sterilization (cont. ) Chlorhexidine – 4%-5% (Hibiscrub/Hibitane) - Active against Gram positive organisms & fungi - Poorly active against Gram negative organisms - Non toxic - Good residual activity - Pre operative skin disinfection/Surgical hand washing - Savlon – 1. 5% Chlorhexidine + 15% Cetrimide Poor bactericidal activity

Disinfection & Sterilization (cont. ) Chlorhexidine – 4%-5% (Hibiscrub/Hibitane) - Active against Gram positive organisms & fungi - Poorly active against Gram negative organisms - Non toxic - Good residual activity - Pre operative skin disinfection/Surgical hand washing - Savlon – 1. 5% Chlorhexidine + 15% Cetrimide Poor bactericidal activity

Principles of disinfection Objects that cannot be heat-treated, should be disinfected by chemicals Clean the article before disinfection Dilution of concentrates should be accurately measured Consider the range of activity, toxicity and cost Beware of inactivating substances Use fresh disinfectant solutions Use clean containers Never 'Top up' disinfection containers Item should kept in contact with the disinfectant for the required time period and no longer than that 35

Chemical disinfectants… Factors affecting the effectiveness/usefulness of chemical disinfection Range of activity Satisfactory contact Concentration p. H- alkaline for gluta. and acid for phenols Neutralization- organic matter, hard water, soap Stability Speed of action- high-hypochlorite Cost Potency- high, intermediate, low Lack of adverse effects 36

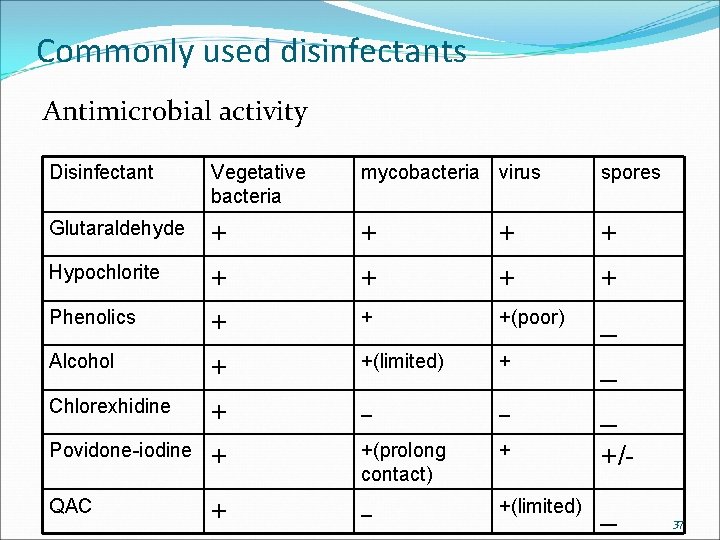
Commonly used disinfectants Antimicrobial activity Disinfectant Vegetative bacteria mycobacteria virus spores Glutaraldehyde + + Hypochlorite + + Phenolics + + +(poor) _ Alcohol + +(limited) + _ Chlorexhidine + _ _ _ Povidone-iodine + +(prolong contact) + +/- QAC + _ +(limited) _ 37

Chemical agents used as high-level disinfectants High level disinfectant Advantages Disadvantages Glutaraldehyde (2%) Relatively inexpensive Excellent material compatibility Needs activation Respiratory irritation Allergic contact dermatitis Irritating odor Hydrogen peroxide (3%) No activation required Enhance removal of organic matter and organisms No odor or irritation Corrosive Eye damage with contact Orthophthalaldehyde (0. 55%) Fast acting No activation required Good material compatibility No odor Stains skin, MM, clothing and environmental surfaces Expensive Eye irritation Peracetic acid (0. 2 -0. 35%) Good material compatibility Fully automated system Environmental friendly by-products Effective in the presence of organic matter Expensive Unstable Small no. of items only per cycle Serious eye and skin damage on contact 38

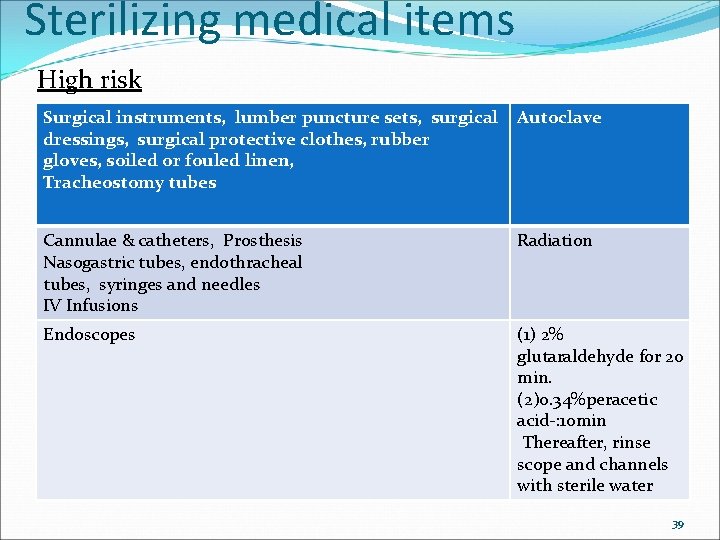
Sterilizing medical items High risk Surgical instruments, lumber puncture sets, surgical Autoclave dressings, surgical protective clothes, rubber gloves, soiled or fouled linen, Tracheostomy tubes Cannulae & catheters, Prosthesis Nasogastric tubes, endothracheal tubes, syringes and needles IV Infusions Radiation Endoscopes (1) 2% glutaraldehyde for 20 min. (2)0. 34%peracetic acid-: 10 min Thereafter, rinse scope and channels with sterile water 39

Intermediate risk -High level disinfection Thermometer Wipe with 70% alcohol swab & store dry Vaginal speculem Tongue depressor Proctoscope Wash with detergent & water and boil for 20 minutes (In high risk patients - autoclave) Respiraotry equipment; Eg: Masks Wash thoroughly Dry & wipe over with 70% alcohol Airways Ambu bag Tubing Disinfect by heat or using 2% glutaraldehyde for 30 min. After immersing in glutaraldehyde, rinse thoroughly in sterile water Nebulizer Use sterile water for nebulization Clean with detergent and hot water after each patient. Tubing can be immersed in 2% glutaraldehyde for 30 min. 40

Low risk- cleaning and low level disinfection Floors Mop with hot water & detergent Walls Clean with hot water & detergent Cutlery & crockery Liquid detergent & hot water Linen All hospital laundry must be heat disinfected Bed pans, urinals Empty the contents into toilet. Fill the utensil with phenolics, leave for 5 min. & wash with water 41

Management of a blood spill Wear heavy duty gloves Soak the spill with an absorbent material Cover with 1% Sodium hypochlorite Leave for 20 minutes Clean 42

Take home message In failure of sterilization and disinfection § § § Increase morbidity and mortality Longer hospital stay Expensive antibiotics Increase work load- doctors, nurses, laboratory Extra cost to the state! Apply your knowledge appropriately 43

THANK YOU 44