Central Service Association Of Ontario Fahrenheit 270 Failure






































- Slides: 61

Central Service Association Of Ontario Fahrenheit 270 Failure Modes and Effects Analysis



ISMP CANADA Vision § Independent nonprofit Canadian organization § Established for: n n the collection and analysis of medication error reports and the development of recommendations for the enhancement of patient safety. § Serves as a national resource for promoting safe medication practices throughout the health care community in Canada.

ISMP Canada Mission: § Committed to the safe use of medication through improvement in drug distribution and drug delivery system design. § Collaborate with healthcare practitioners and institutions, schools, professional organizations, pharmaceutical industry and regulatory & government agencies to provide education about adverse drug events and their prevention

Objectives n n n To introduce the principle and application of FMEA tool/process To discuss its application in healthcare facilities To briefly review the FMEA process

Failure Mode and Effects Analysis § § § ISMP Canada one day workshop 4 exercises of 45 – 60 minutes Practce on your own issues

Human Factors Engineering 101 HFE: a discipline concerned with design of systems, tools, processes, machines that take into account human capabilities, limitations, and characteristics

Human Factors Engineering § Research and practical applications designed to improve the interface of humans with systems § Develops practical design principles that account for the psychological and physical characteristics of people

Human Factors Engineering Principles § § § Simplify key processes Standardize work processes Anticipate that human make errors Use checklists Improve label design Promote effective team functioning

Constraint: Hydromorphone 10 mg was removed

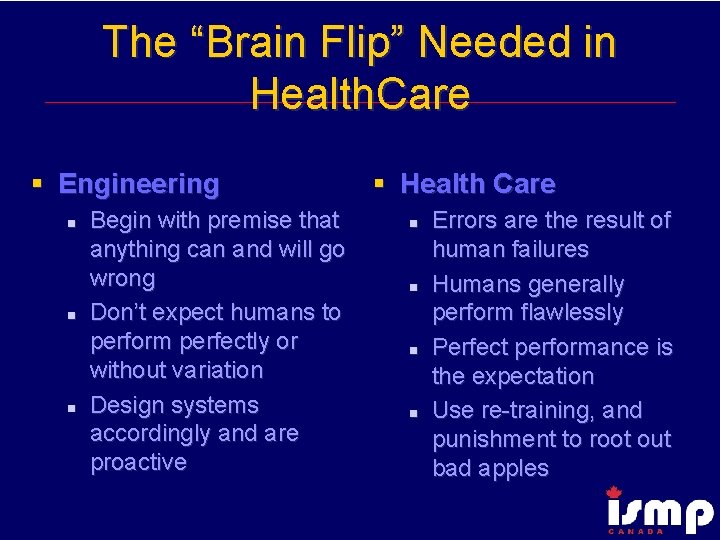
The “Brain Flip” Needed in Health. Care § Engineering n n n Begin with premise that anything can and will go wrong Don’t expect humans to perform perfectly or without variation Design systems accordingly and are proactive § Health Care n n Errors are the result of human failures Humans generally perform flawlessly Perfect performance is the expectation Use re-training, and punishment to root out bad apples

Human Factors – Guiding Principle Fit the task or tool to the human, not the other way around.

High Reliability Organizations (HRO) Characteristics § Collective preoccupation with the possibility of failure” § Expect to make errors and train their workforce to recognize and recover from them § Continual rehearsal of familiar scenarios of failure


FMEA Definition § FMEA is a team-based systematic and proactive approach for identifying the ways that a process or design can fail, why it might fail, the effects of that failure and how it can be made safer. § FMEA focuses on how and when a system will fail, not IF it will fail.

Why FMEA? § § § Brings analysis logic into the hospital Is a proactive approach for quality and safety Initiates system fixes before a patient dies or is injured Makes systems more “robust” and enhances performance Makes systems more “fault tolerant” Focuses on systems, not individuals

Everyday FMEA Yes No § Do you take actions to prevent yourself from being late to work? § Do you take a ‘shortcut’ when you see traffic building up in a familiar place? § Do you try to distinguish ‘big problems’ from ‘little problems’? § Do you see the possibility of eliminating some problems, but need a better way to show that to people?

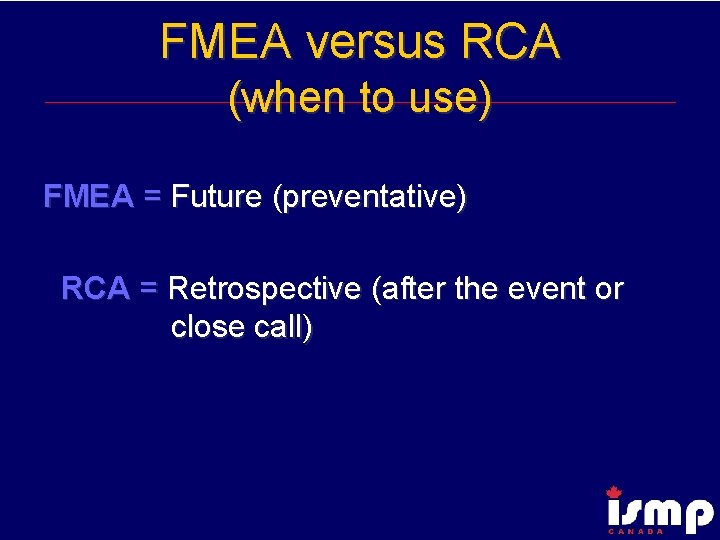
FMEA versus RCA (when to use) FMEA = Future (preventative) RCA = Retrospective (after the event or close call)

FMEA Origins § FMEA in use more than 40 years beginning in aerospace in the 1960 s § 1970 s and 1980 s used in other fields such as nuclear power, aviation, chemical, electronics and food processing fields ( High Reliability Organizations) § Automotive industry requires it from suppliers, reducing the after-the-fact corrective actions

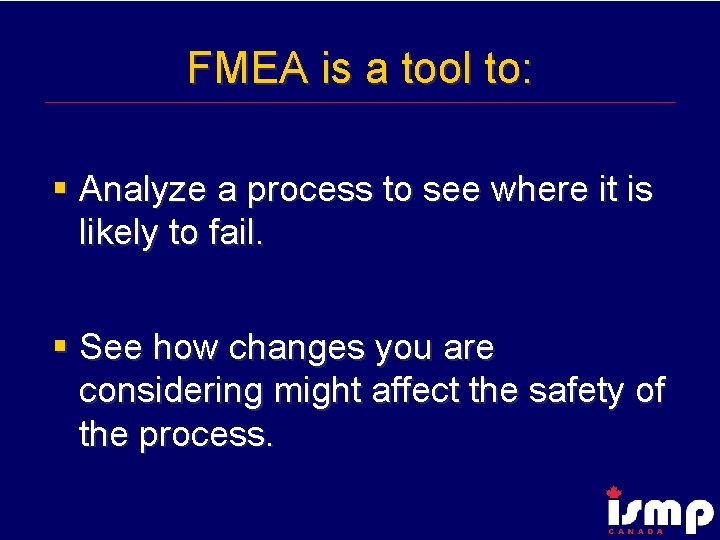
FMEA is a tool to: § Analyze a process to see where it is likely to fail. § See how changes you are considering might affect the safety of the process.

CCHSA Patient Safety Goals 2005 § Create a culture of safety within the organization n The organization has in place a formal team/committee whose sole focus is patient safety, and that does one proactive RCA or FMEA a year.

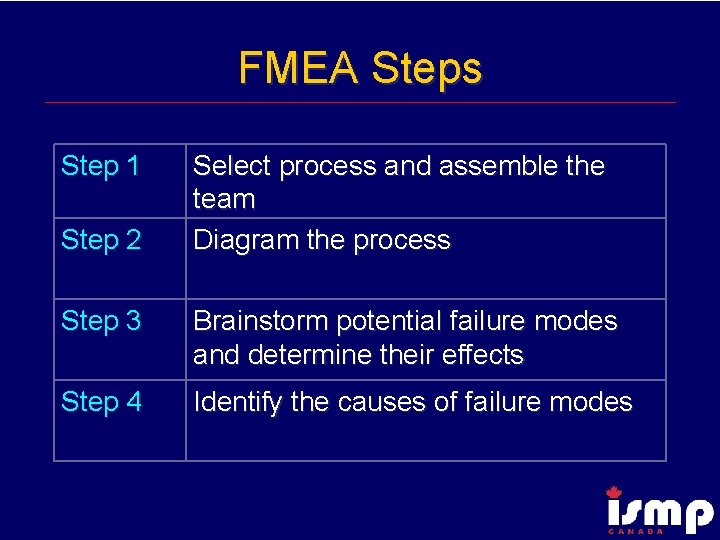
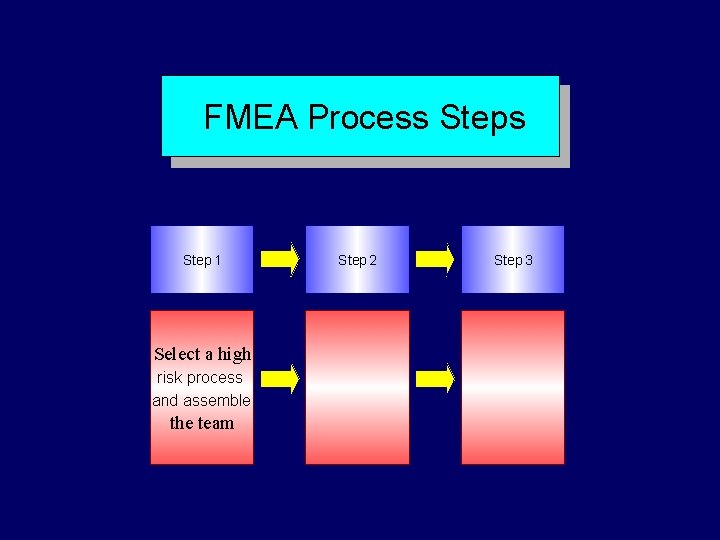
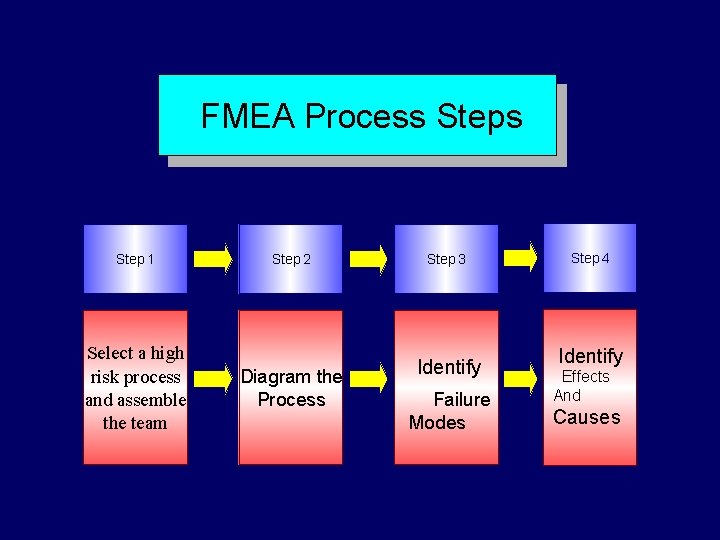
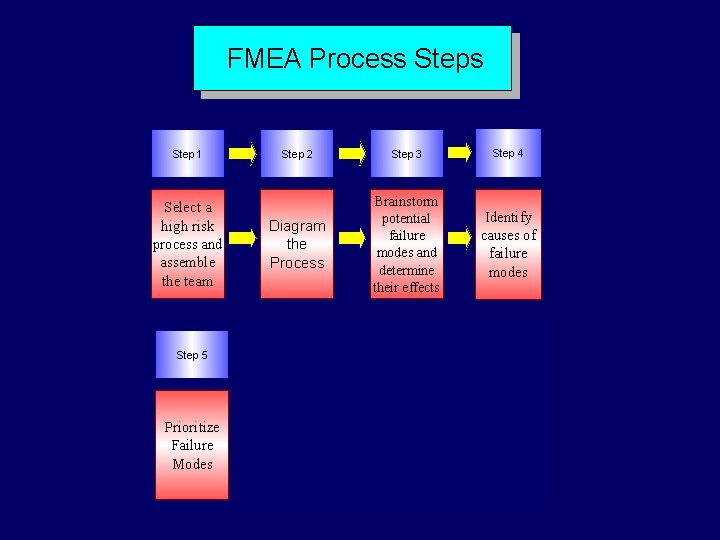
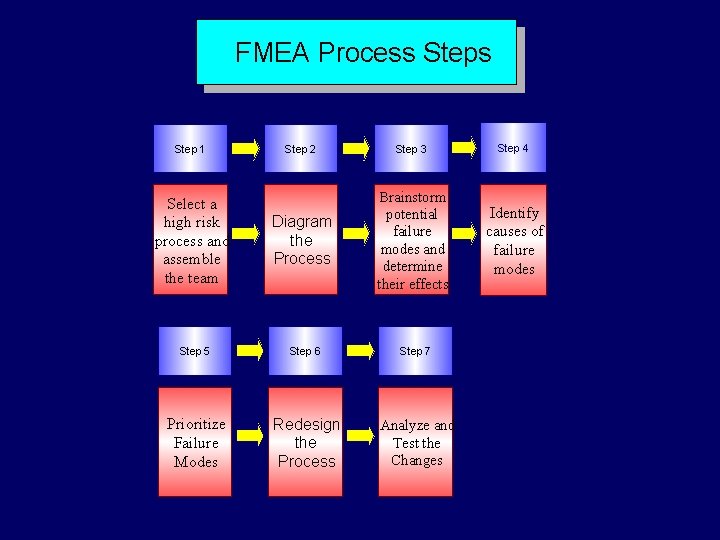
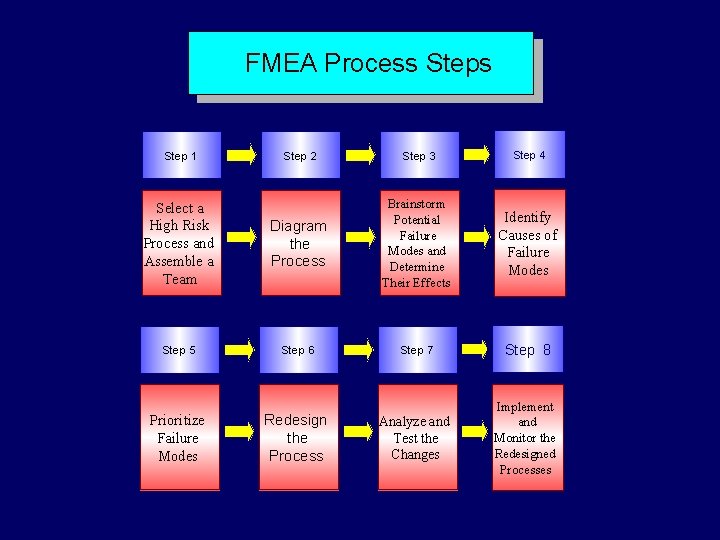
FMEA Steps Step 1 Step 2 Select process and assemble the team Diagram the process Step 3 Brainstorm potential failure modes and determine their effects Step 4 Identify the causes of failure modes

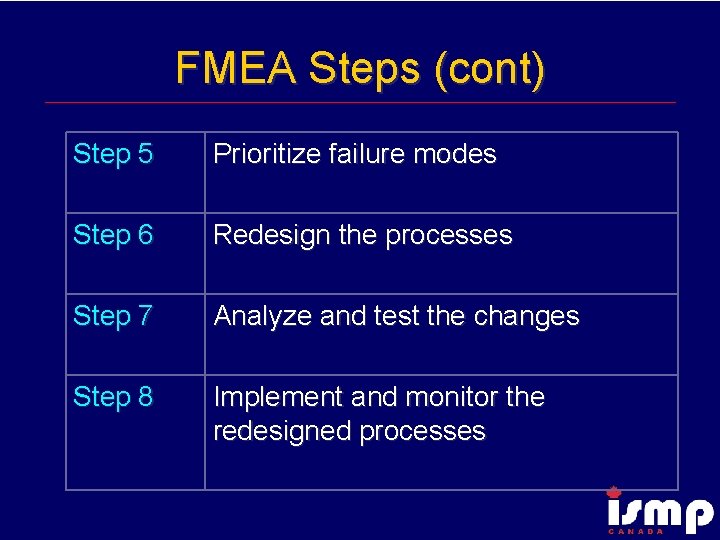
FMEA Steps (cont) Step 5 Prioritize failure modes Step 6 Redesign the processes Step 7 Analyze and test the changes Step 8 Implement and monitor the redesigned processes

FMEA Process Step 1 Select a high risk process and assemble the team Step 2 Step 3

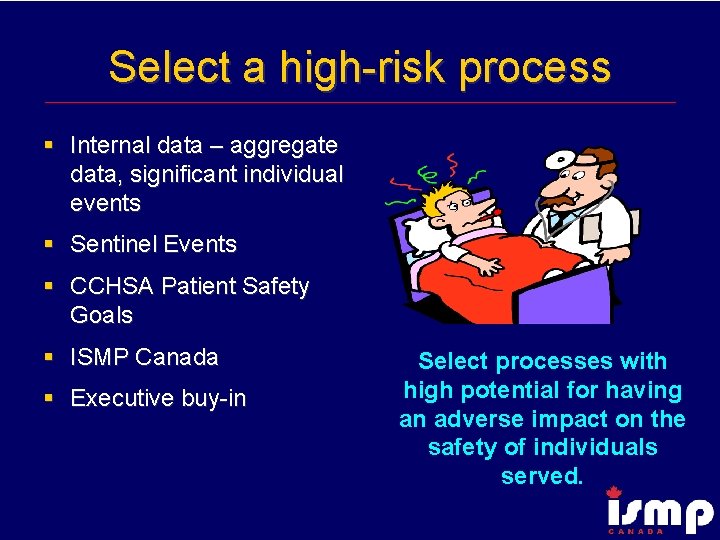
Select a high-risk process § Internal data – aggregate data, significant individual events § Sentinel Events § CCHSA Patient Safety Goals § ISMP Canada § Executive buy-in Select processes with high potential for having an adverse impact on the safety of individuals served.

High Risk Processes - Definition § Those processes in which a failure of some type is most likely to jeopardize the safety of the individuals served by the health care organization. Such process failures may result in a sentinel event.

High Risk Processes - Examples § Medication Use § Operative and other procedures § Blood use and blood components § Restraints § Infection control § Care provided to high-risk population § Emergency or resuscitation care

Assemble a team § § Leader Facilitator Scribe / Recorder Process experts n Include all areas involved in the process § “Outsider” /Naïve person § 6 -10 optimal number

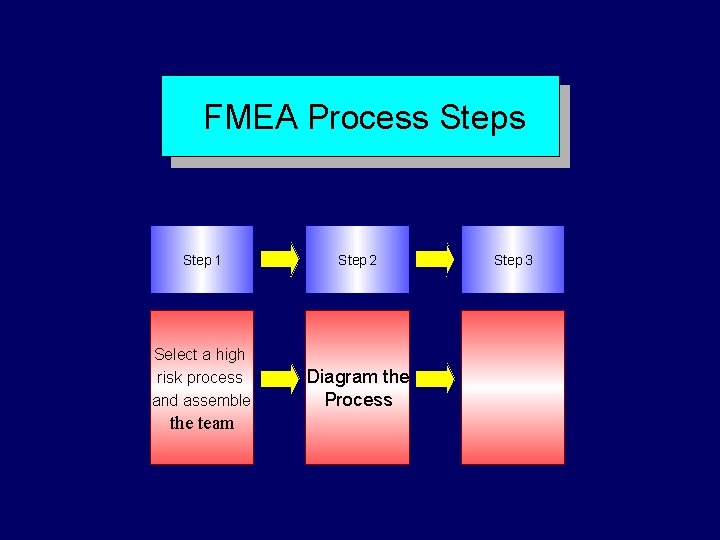
FMEA Process Step 1 Step 2 Select a high risk process and assemble Diagram the Process the team Step 3

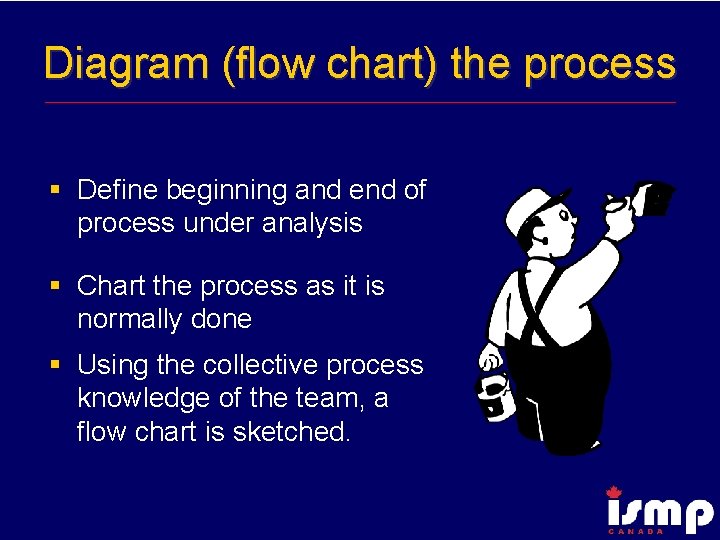
Diagram (flow chart) the process § Define beginning and end of process under analysis § Chart the process as it is normally done § Using the collective process knowledge of the team, a flow chart is sketched.

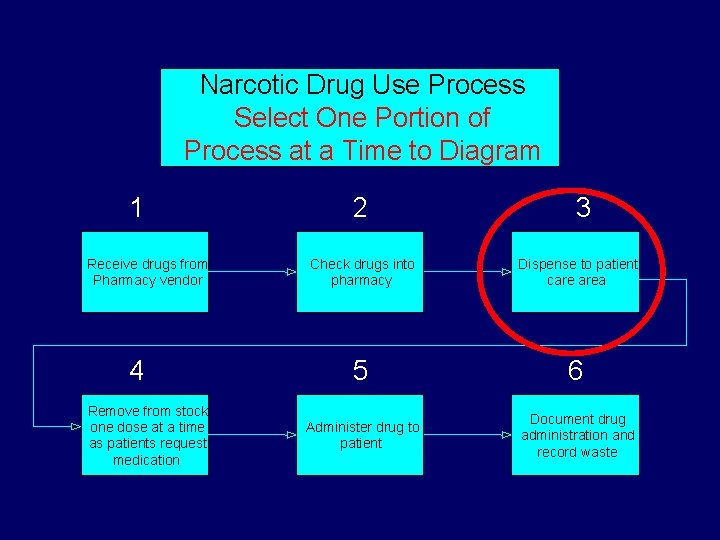
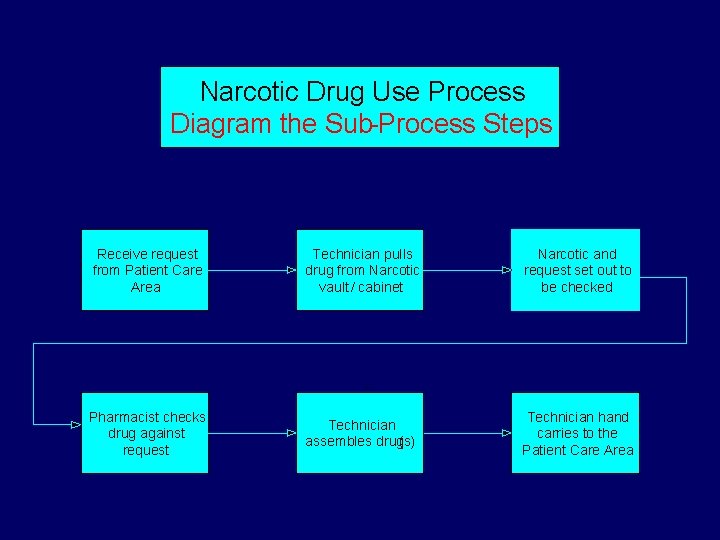
Why diagram the process? § Diagrams clarify things between members § Narrows the topic – goes from broad topic e. g. narcotic use process to narrow topic e. g. morphine removed from narcotic drawer

Narcotic Drug Use Process Number Basic Steps 1 Receive drugs from Pharmacy vendor 4 Remove from stock one dose at a time as patients request medication 2 3 Check drugs into pharmacy Dispense to patient care area 5 6 Administer drug to patient Document drug administration and record waste

Narcotic Drug Use Process Select One Portion of Process at a Time to Diagram 1 Receive drugs from Pharmacy vendor 4 Remove from stock one dose at a time as patients request medication 2 3 Check drugs into pharmacy Dispense to patient care area 5 6 Administer drug to patient Document drug administration and record waste

Narcotic Drug Use Process Diagram the Sub-Process Steps Receive request from Patient Care Area Technician pulls drug from Narcotic vault / cabinet Narcotic and request set out to be checked Pharmacist checks drug against request Technician assembles drug(s) Technician hand carries to the Patient Care Area

FMEA Process Step 1 Select a high risk process and assemble the team Step 2 Diagram the Process Step 3 Brainstorm potential failure modes

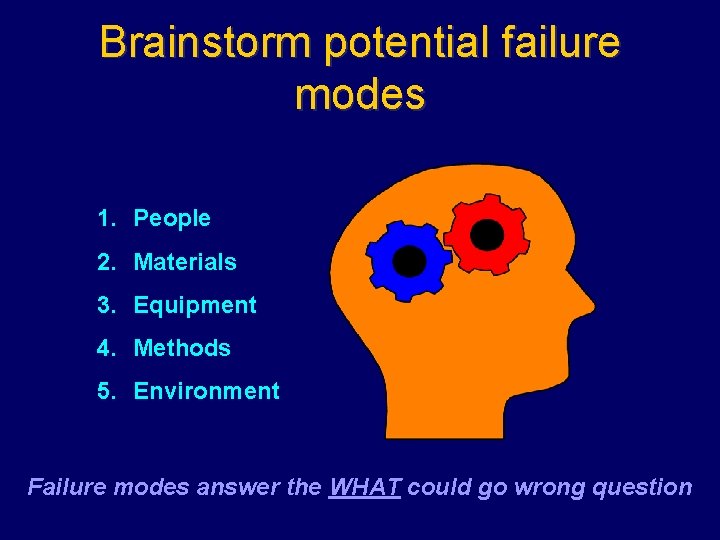
Brainstorm potential failure modes 1. People 2. Materials 3. Equipment 4. Methods 5. Environment Failure modes answer the WHAT could go wrong question

Handy Hints ü Failure Modes are the WHATs that could go wrong ü Failure Mode Causes are the “WHY”s ü May be more than one cause for each failure

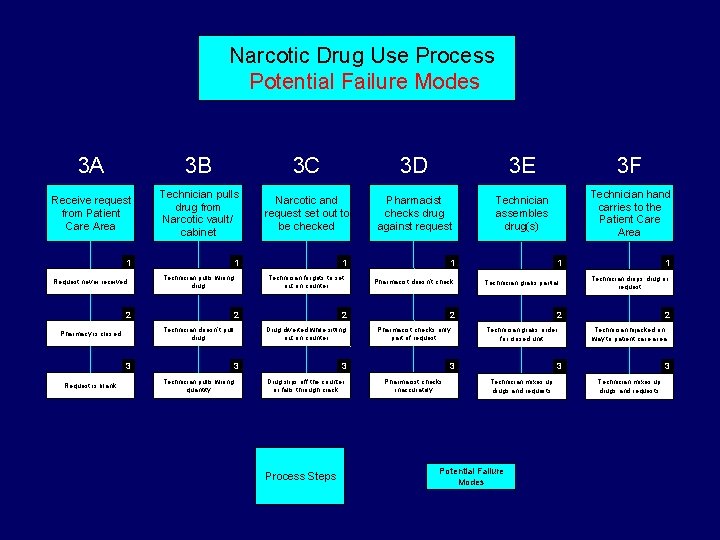
Narcotic Drug Use Process Potential Failure Modes 3 A 3 B 3 C 3 D 3 E 3 F Receive request from Patient Care Area Technician pulls drug from Narcotic vault/ cabinet Narcotic and request set out to be checked Pharmacist checks drug against request Technician assembles drug(s) Technician hand carries to the Patient Care Area 1 Request never received 1 Technician pulls wrong drug 2 2 Technician doesn’t pull drug Pharmacy is closed 3 Request is blank 1 Technician forgets to set out on counter Pharmacist doesn’t check 2 Drug diverted while sitting out on counter 3 Technician pulls wrong quantity 1 Process Steps Technician grabs partial 2 Pharmacist checks only part of request 3 Drug slips off the counter or falls through crack 1 Technician drops drug or request 2 Technician grabs order for closed unit 3 Pharmacist checks inaccurately 1 2 Technician hijacked on way to patient care area 3 Technician mixes up drugs and requests Potential Failure Modes 3 Technician mixes up drugs and requests

FMEA Process Step 1 Select a high risk process and assemble the team Step 2 Diagram the Process Step 3 Identify Failure Modes Step 4 Identify Effects And Causes

Effects of the Failure Modes § Review each failure mode and identify the effects of the failure should it occur § May be 1 effect or > 1 § Must be thorough because it feeds into the risk rating § If failure occurs, then what are the consequences

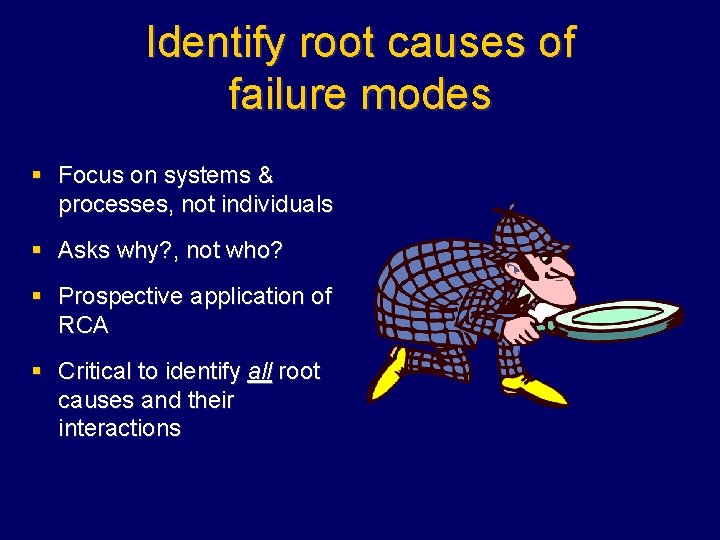
Identify root causes of failure modes § Focus on systems & processes, not individuals § Asks why? , not who? § Prospective application of RCA § Critical to identify all root causes and their interactions

Single Point Weakness • A step so critical that its failure will result in a system failure or adverse event • Single point weaknesses and existing control measures “modify” the scoring Ø Ø Single point weaknesses should all be acted upon IF effective control measures are in place, it would cancel the need to take further action (risk is mitigated)

FMEA Process Step 1 Select a high risk process and assemble the team Step 5 Prioritize Failure Modes Step 2 Step 3 Step 4 Diagram the Process Brainstorm potential failure modes and determine their effects Identify causes of failure modes

Prioritize failure modes § Score severity of effect of failure mode § Score frequency of occurrence of failure mode § Score likelihood of detection (detectability) of failure prior to the impact of the effect being realized

Severity The seriousness and severity of the effect (to the process or system or patient) of a failure if it should occur. Score based upon a “reasonable worst case scenario”

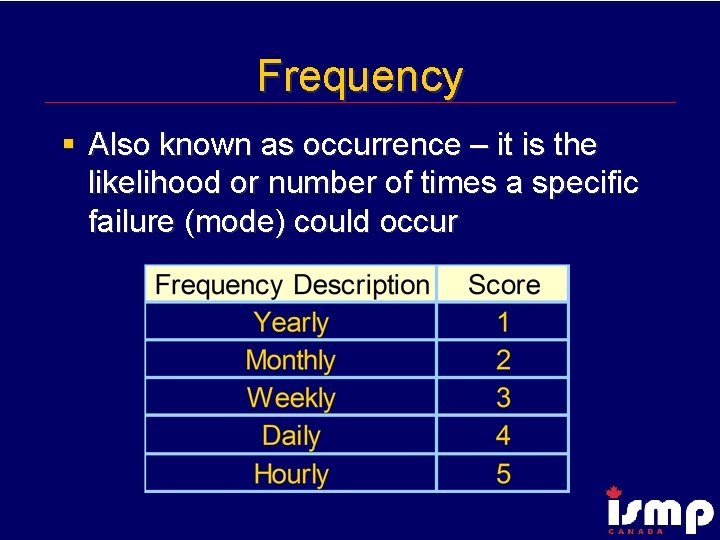
Frequency § Also known as occurrence – it is the likelihood or number of times a specific failure (mode) could occur

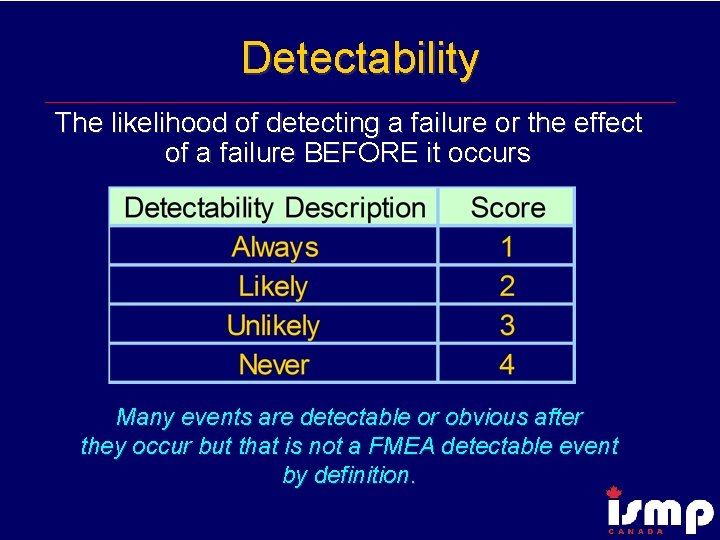
Detectability The likelihood of detecting a failure or the effect of a failure BEFORE it occurs Many events are detectable or obvious after they occur but that is not a FMEA detectable event by definition.

Examples of Detectability § § Break away locks Emergency drug boxes with pop up pin Ampoules Low battery alarm

Calculate the Risk Priority Number § Determine the impact of the failure on the patient or the system using the severity, frequency and detectability parameters § Multiply three scores to obtain a Risk Priority Number (RPN) or Criticality Index (CI) § Also assign priority to those with a high severity score even though the RPN may be relatively low RPN = Severity x Occurrence x Detection

FMEA Process Step 2 Step 3 Step 4 Select a high risk process and assemble the team Diagram the Process Brainstorm potential failure modes and determine their effects Identify causes of failure modes Step 5 Step 6 Prioritize Failure Modes Redesign the Process Step 1

Redesign the process § Apply strategies to decrease occurrence, decrease severity, or increase detection § Goal: prevent harm to the patient § Simplification, automation, standardization, fail-safe mechanisms, forcing functions, redundancy

Evaluating Redesign Options § Don’t just pick training and policy development. They are basic actions but not very strong or long lasting § Go for the permanent fixes when possible § Elimination of the step or the function is a very strong action § Most actions are really controls on the system § Sometimes your team might have to accept some of the failure modes as “un-fixable”

Rank Order of Error Reduction Strategies 1. 2. 3. 4. 5. 6. 7. 8. Forcing functions and constraints Automation and computerization Simplify, standardize and differentiate Reminders, check lists and double check systems Rules and policies Education Information Punishment (no value)

FMEA Process Step 2 Step 3 Step 4 Select a high risk process and assemble the team Diagram the Process Brainstorm potential failure modes and determine their effects Identify causes of failure modes Step 5 Step 6 Step 7 Prioritize Failure Modes Redesign the Process Analyze and Test the Changes Step 1

Analyze and test the changes § Conduct FMEA of redesigned process § Use simulation testing whenever possible § Conduct pilot testing in one area or one section

FMEA Process Step 1 Select a High Risk Process and Assemble a Team Step 5 Prioritize Failure Modes Step 2 Step 3 Step 4 Diagram the Process Brainstorm Potential Failure Modes and Determine Their Effects Identify Causes of Failure Modes Step 6 Step 7 Step 8 Analyze and Test the Changes Implement and Monitor the Redesigned Processes Redesign the Process

Implement and monitor the redesigned process § Communicate reasons for process changes § Find change agents § Define process and outcome measures § Share results § Monitor over time

Gains using FMEA § § § Safety minded culture Proactive problem resolution Robust systems Fault tolerant systems Lower waste and higher quality

‘Emphasis on prevention may reduce risk of harm to both patients and staff. ’ Failure Modes and Effects Analysis (FMEA), IHI and Quality Health Care. org, 2003

References § Mc. Dermott- The Basics of FMEA § Stamatis – Failure Mode Effect Analysis: FMEA from Theory to Execution (2 nd ed) § JCAHO – Failure Mode and Effects Analysis in Health Care. Proactive Risk Reduction § Manasse, Thompson (Lin, Burkhardt) -Logical Application of Human Factors In Process and Equipment Design (in press).
 California central service association
California central service association Ventricular escape rhythm
Ventricular escape rhythm Failure to sense
Failure to sense Moderately ductile fracture
Moderately ductile fracture Arc flash training ontario
Arc flash training ontario Paro vacation rules
Paro vacation rules Ontario search and rescue volunteer association
Ontario search and rescue volunteer association Ontario association of physiotherapists
Ontario association of physiotherapists Ontario buyers association
Ontario buyers association Ontario disc sports association
Ontario disc sports association London ambulance service failure
London ambulance service failure Central idea vs theme
Central idea vs theme Conflicts in fahrenheit 451
Conflicts in fahrenheit 451 90 degree counterclockwise rotation
90 degree counterclockwise rotation 270 rotation
270 rotation 270 towin
270 towin Legea 270 din 2018 cu modificari
Legea 270 din 2018 cu modificari Loran
Loran Hcf of 90
Hcf of 90 270 towin.com
270 towin.com Umich eecs 215
Umich eecs 215 Formula for 270 degree rotation clockwise
Formula for 270 degree rotation clockwise Jika sin a = 3/5 a sudut pada kuadran ii maka cos a =
Jika sin a = 3/5 a sudut pada kuadran ii maka cos a = Csc 270
Csc 270 Primer cuadrante
Primer cuadrante 360 270
360 270 Zodiac fastroller 380
Zodiac fastroller 380 ángulos que difieren en 270
ángulos que difieren en 270 Arah mata angin primer
Arah mata angin primer 0 90 180 360
0 90 180 360 270 to win.com
270 to win.com Figuras asimetricas
Figuras asimetricas Ics
Ics Csc of 270
Csc of 270 Class complex
Class complex Eecs 270
Eecs 270 Express 45/270 as a fraction reduced to its lowest terms
Express 45/270 as a fraction reduced to its lowest terms 7 more rotations
7 more rotations Sdt ultrasonic
Sdt ultrasonic Bfs time complexity
Bfs time complexity Kompasskurs 270 grader
Kompasskurs 270 grader Sinus 270
Sinus 270 Fsms 2020
Fsms 2020 Tiga kodi baju dibeli dengan harga 400
Tiga kodi baju dibeli dengan harga 400 Csc(270)
Csc(270) Csc 270
Csc 270 Cs 270 csu
Cs 270 csu Convertir 120 grados a radianes
Convertir 120 grados a radianes Central bucks athletic association
Central bucks athletic association Down syndrome association of central oklahoma
Down syndrome association of central oklahoma Melyne strickland
Melyne strickland Cordilleran vegetation
Cordilleran vegetation Softball ontario rules
Softball ontario rules Leadership framework ontario
Leadership framework ontario Derecho storm ontario
Derecho storm ontario Long lot settlement pattern
Long lot settlement pattern Ontario qualifications framework level 12
Ontario qualifications framework level 12 Ontariocolleges
Ontariocolleges Health and safety act ontario
Health and safety act ontario National student loans center
National student loans center Mtcu thunder bay
Mtcu thunder bay Cadre de leadership de l'ontario
Cadre de leadership de l'ontario