Cement Hydration using Isothermal Calorimetry David Kirby Naresh

Cement Hydration using Isothermal Calorimetry David Kirby Naresh Tadisina

Outline �Objectives �Introduction to Cements �Experimental Procedures �Experimental Results �Modeling Approach �Model Results �Activation Energy Calculation �Conclusion �References
Research objective �To determine the kinetic parameters for cement hydration using the Avrami equation and the grain boundary nucleation and growth model. �To determine the temperature dependence of rate constant � To determine which equation can best model the hydration of cement.
Introduction q. Cement: �Major raw material for concrete and mortar �Dry, hydroscopic material �Powder form �To bind the aggregate materials of concrete. �Addition of water and aggregates the cement mixture is referred to as concrete. �More than 1 billion tons of cement consumed annually �Portland cement is most common type of cement in general usage.
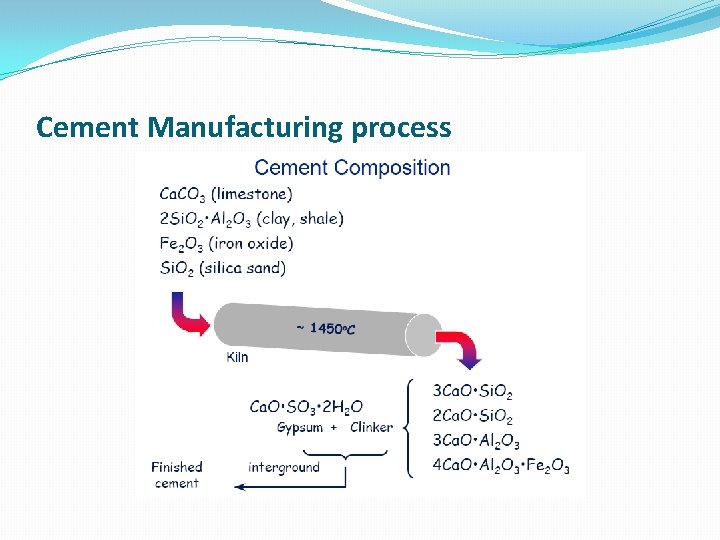
Cement Manufacturing process

ASTM standard specifications for Portland cement �ASTM Type: I Normal II Moderate sulfate resistance or heat III High early strength IV Low heat of hydration V High sulfate resistance
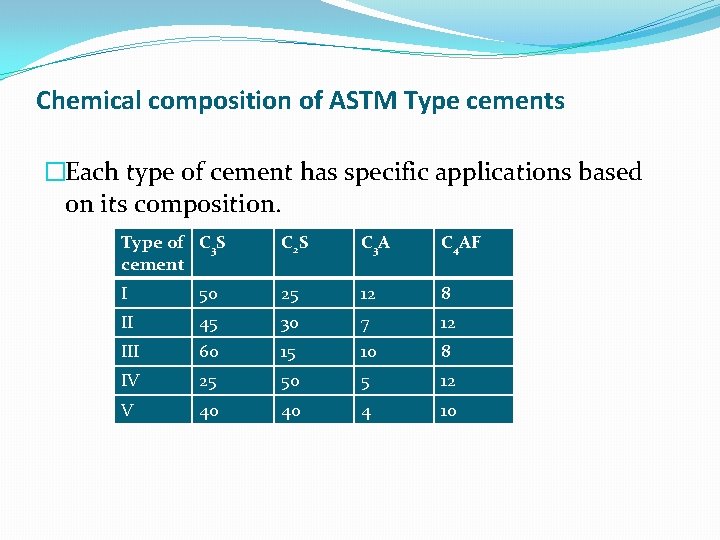
Chemical composition of ASTM Type cements �Each type of cement has specific applications based on its composition. Type of C 3 S cement C 2 S C 3 A C 4 AF I 50 25 12 8 II 45 30 7 12 III 60 15 10 8 IV 25 50 5 12 V 40 40 4 10
Cement Hydration �Chemical combination of cement and water to form hydration products. �What happens when water is added to cement? �Dissolution of cement grains �Growing ionic concentration in “water” (now a solution) �Formation of compounds in solution �After reaching a saturation concentration, compounds precipitate out as solids (“hydration products”) �In later stages, products form on or very near the surface of the anhydrous cement

Cement Hydration(cont’d) �Formation of hydration products over time leads to: �Stiffening(loss of workability) �Setting (solidification) �Hardening (strength gain) �The hydration rates of 4 major constituents of cement vary considerably, so these properties will vary with cement composition. �Cement hydration is an exothermic reaction. �Usually does not proceed to 100% completion.
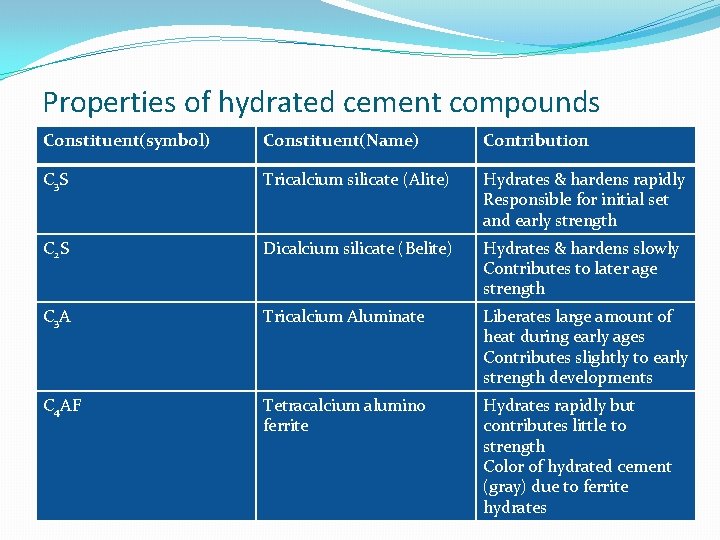
Properties of hydrated cement compounds Constituent(symbol) Constituent(Name) Contribution C 3 S Tricalcium silicate (Alite) Hydrates & hardens rapidly Responsible for initial set and early strength C 2 S Dicalcium silicate (Belite) Hydrates & hardens slowly Contributes to later age strength C 3 A Tricalcium Aluminate Liberates large amount of heat during early ages Contributes slightly to early strength developments C 4 AF Tetracalcium alumino ferrite Hydrates rapidly but contributes little to strength Color of hydrated cement (gray) due to ferrite hydrates
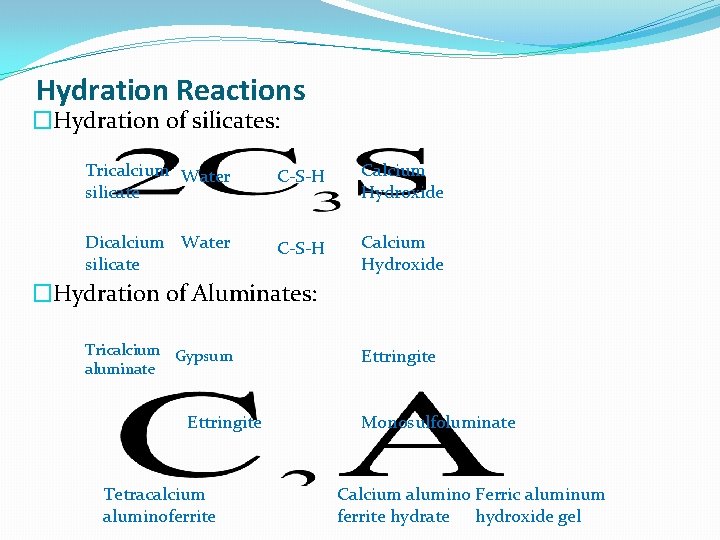
Hydration Reactions �Hydration of silicates: Tricalcium Water silicate C-S-H Calcium Hydroxide Dicalcium Water silicate C-S-H Calcium Hydroxide �Hydration of Aluminates: Tricalcium Gypsum aluminate Ettringite Tetracalcium aluminoferrite Ettringite Monosulfoluminate Calcium alumino Ferric aluminum ferrite hydrate hydroxide gel

Stages Of Cement Hydration 1. Initial Dissolution 2. Induction Period 3. Acceleration 4. Deceleration 5. Steady-State (diffusion)
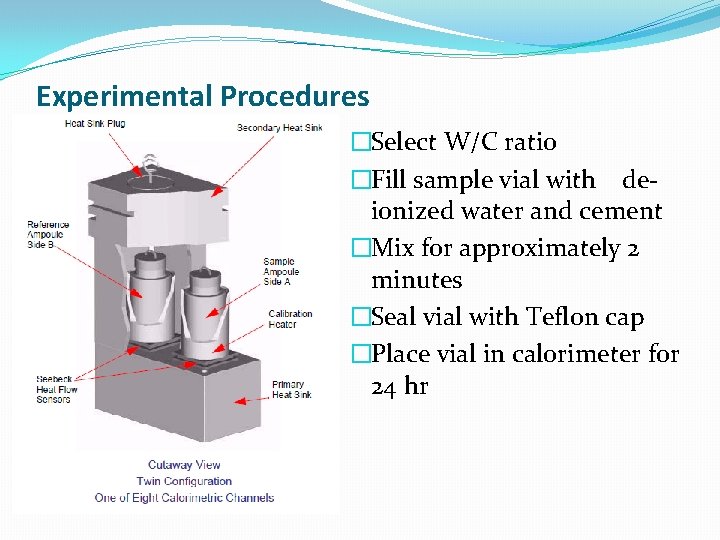
Experimental Procedures �Select W/C ratio �Fill sample vial with deionized water and cement �Mix for approximately 2 minutes �Seal vial with Teflon cap �Place vial in calorimeter for 24 hr
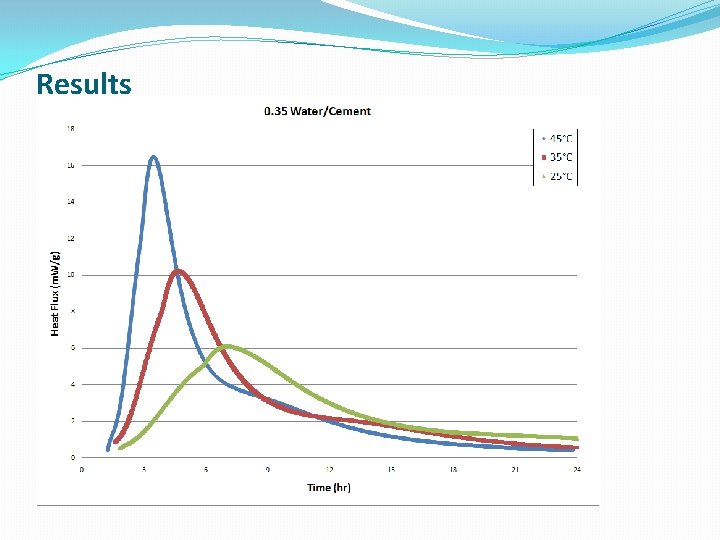
Results
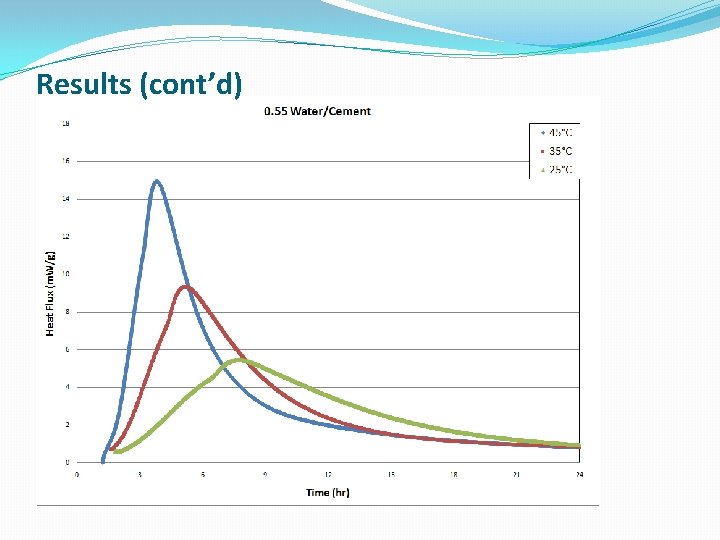
Results (cont’d)
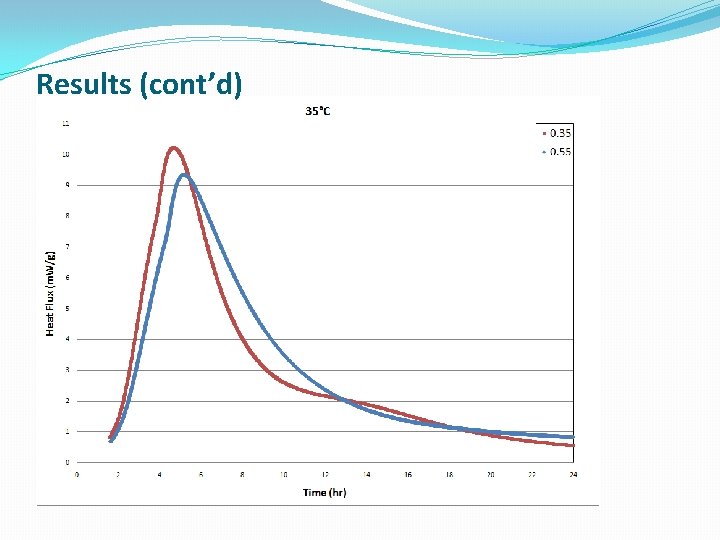
Results (cont’d)
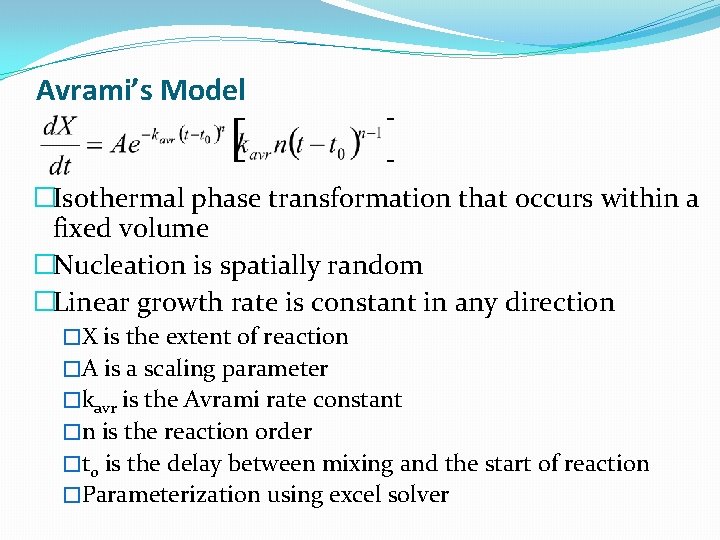
Avrami’s Model �Isothermal phase transformation that occurs within a fixed volume �Nucleation is spatially random �Linear growth rate is constant in any direction �X is the extent of reaction �A is a scaling parameter �kavr is the Avrami rate constant �n is the reaction order �t 0 is the delay between mixing and the start of reaction �Parameterization using excel solver
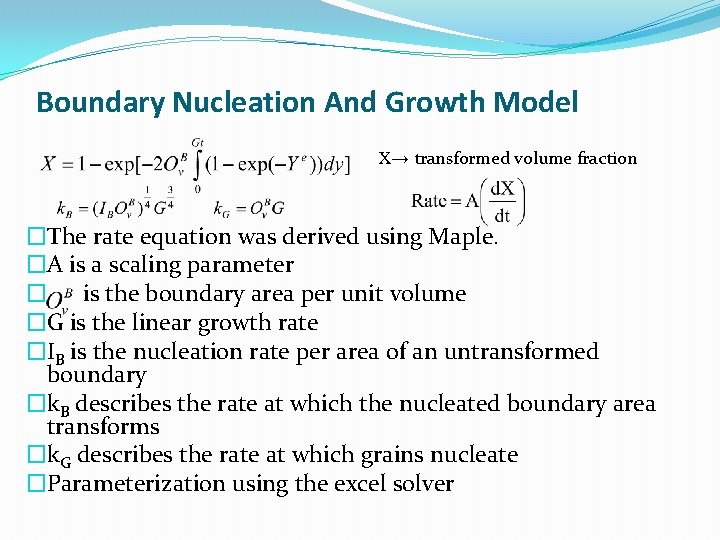
Boundary Nucleation And Growth Model X→ transformed volume fraction �The rate equation was derived using Maple. �A is a scaling parameter � is the boundary area per unit volume �G is the linear growth rate �IB is the nucleation rate per area of an untransformed boundary �k. B describes the rate at which the nucleated boundary area transforms �k. G describes the rate at which grains nucleate �Parameterization using the excel solver
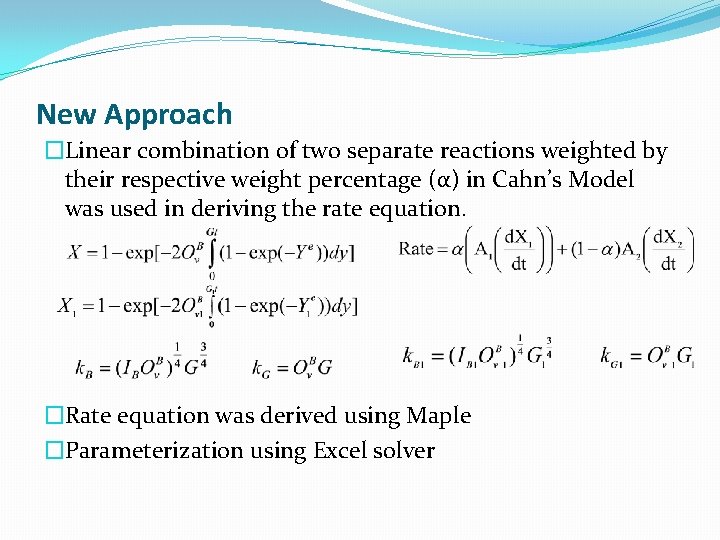
New Approach �Linear combination of two separate reactions weighted by their respective weight percentage (α) in Cahn’s Model was used in deriving the rate equation. �Rate equation was derived using Maple �Parameterization using Excel solver
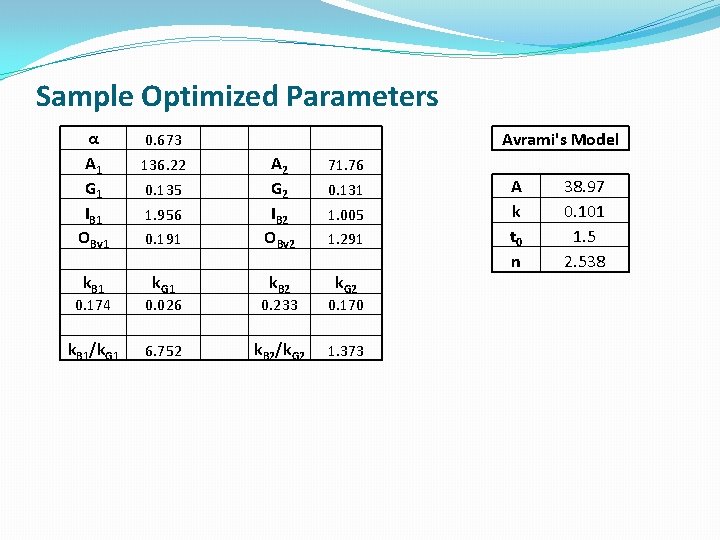
Sample Optimized Parameters α A 1 G 1 IB 1 OBv 1 k. B 1 Avrami's Model 0. 673 71. 76 1. 956 0. 191 A 2 G 2 IB 2 OBv 2 k. G 1 k. B 2 k. G 2 136. 22 0. 135 0. 131 1. 005 1. 291 0. 174 0. 026 0. 233 0. 170 k. B 1/k. G 1 6. 752 k. B 2/k. G 2 1. 373 A k t 0 n 38. 97 0. 101 1. 5 2. 538
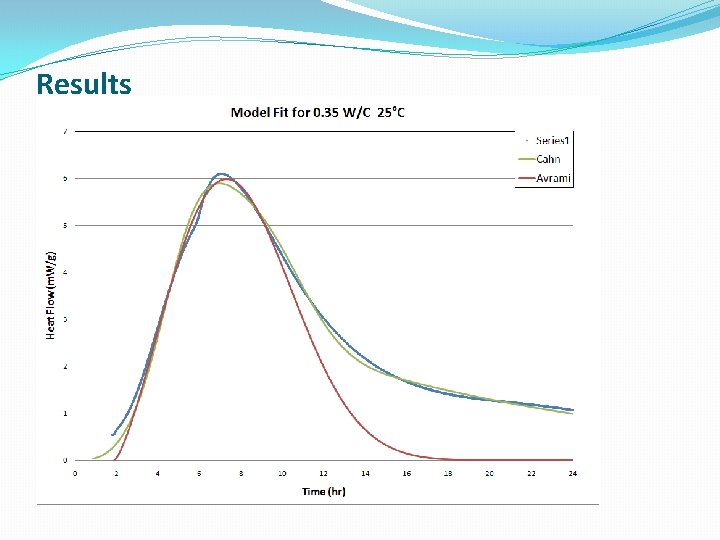
Results
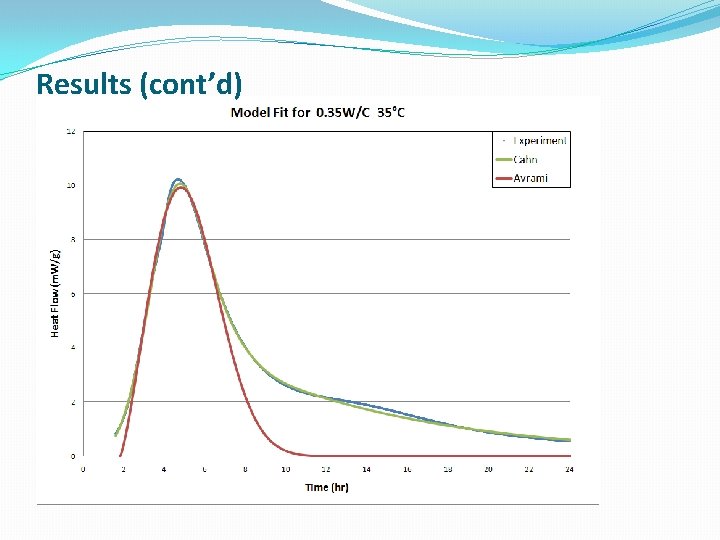
Results (cont’d)
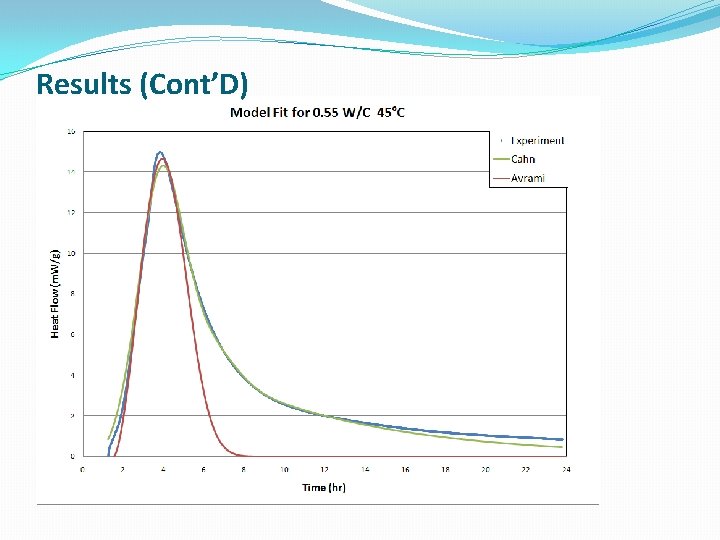
Results (Cont’D)
Temperature dependence of Kinetic parameters �Arhenius equation �Cement hydration is a rate-limited thermally activated process �This type of reaction gives a straight line on the Arrhenius Plot
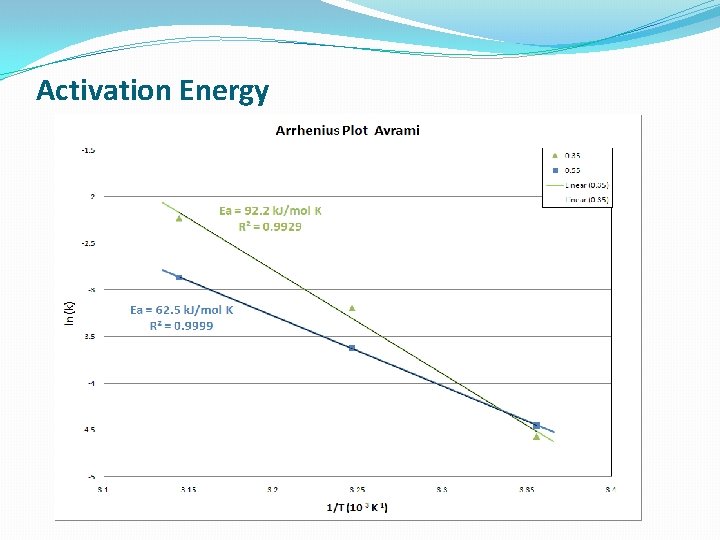
Activation Energy
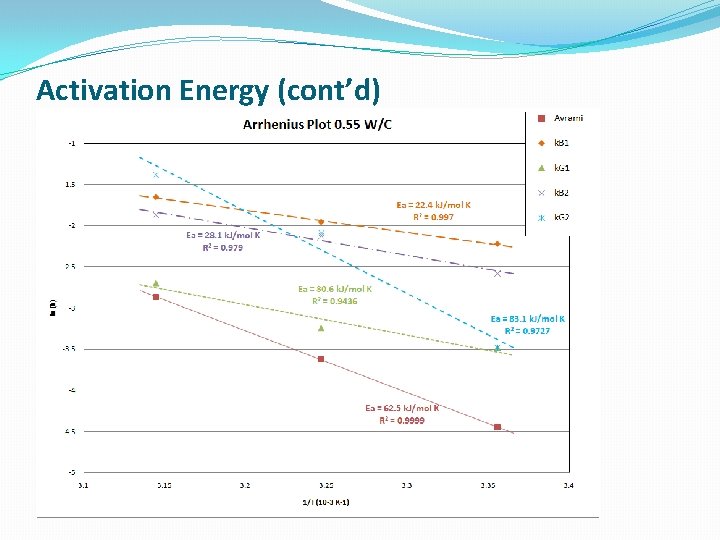
Activation Energy (cont’d)
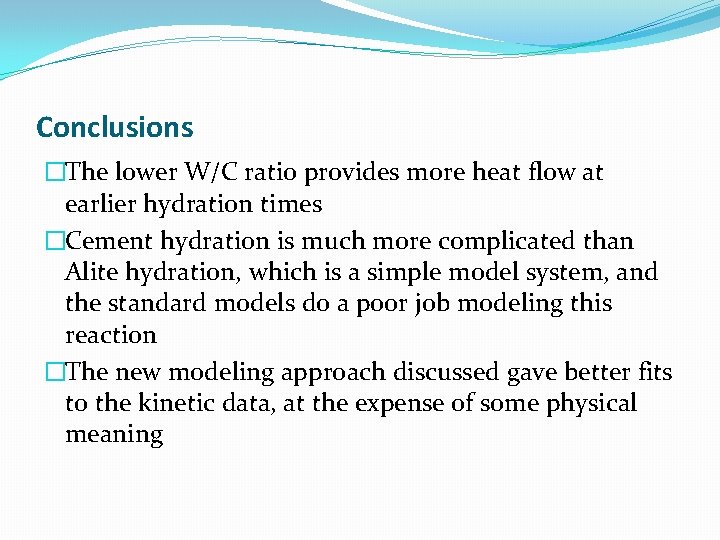
Conclusions �The lower W/C ratio provides more heat flow at earlier hydration times �Cement hydration is much more complicated than Alite hydration, which is a simple model system, and the standard models do a poor job modeling this reaction �The new modeling approach discussed gave better fits to the kinetic data, at the expense of some physical meaning
![References � [1]. J. J. Thomas. , “A New Approach to Modeling the Nucleation References � [1]. J. J. Thomas. , “A New Approach to Modeling the Nucleation](http://slidetodoc.com/presentation_image_h2/e9b20f1c9cc642c656be141823af8f2a/image-28.jpg)
References � [1]. J. J. Thomas. , “A New Approach to Modeling the Nucleation and Growth Kinetics of Tricalcium Silicate Hydration”, J. Am. Ceram. Soc. , 90 [10]3282 -3288 (2007). � [2]. S. Mindess. , J. F. Young. , “Concrete”, Prentice-Hall, Inc. New Jersey 07632 (1981). � [3]. J. W. Bullard. , “A Determination of Hydration Mechanism for Tricalcium Silicate Using a Kinetic Cellular Automaton Model”, J. Am. Ceram. Soc. , 91 [7] 2088 -2097 (2008). � [4]. D. P. Bentz. , “Influence of Water-to-Cement Ratio on Hydration Kinetics: Simple Models Based on Spatial Considerations”, Cement and Concrete Research, Vol. 36, No. 2, PP 238 -244, (2006). � [5]. A. Bezjak. , “Kinetics Analysis of Cement Hydration Including Various Mechanistic Concepts”. 1. Theoretical Development”, Cement and Concrete Research. Vol. 13, pp. 305318, (1983). � [6]. Y. Peng. , W. Hansen. , C. Borgnakke. , I. Pane. , J. C. Roumain. , J. J. Biernakci. , “Thermo-kinetics of cement hydration: temperature effect and activation energy”, International RILEM Symposium on Concrete Science and Engineering: A Tribute to Arnon Bentur. (2004). � [7]. J. W. Cahn. , “The Kinetics of Grain Boundary Nucleated Reactions”, Acta Metall. , 4, 449 -59 (1956). � [8]. O. Levenspiel. , “Chemical Reaction Engineering”, Third Edition, John Wiley & Sons, Inc. (1999

Acknowledgements �Dr. Joseph Biernacki �Dr. Benjamin Mohr �Tiantian Xie �Ch. E 6210 Group
- Slides: 29