Cellular Respiration Organic compounds store energy in their





















- Slides: 69

Cellular Respiration

• Organic compounds store energy in their arrangement of atoms • Fats, CH 2 O protein can all be used as fuel. Traditionally, cellular respiration is studied using glucose as the source. • There are 2 energy-providing (catabolic) pathways – Cellular Respiration AEROBIC! – Fermentation ( partial degradation of sugar without oxygen) ANAEROBIC

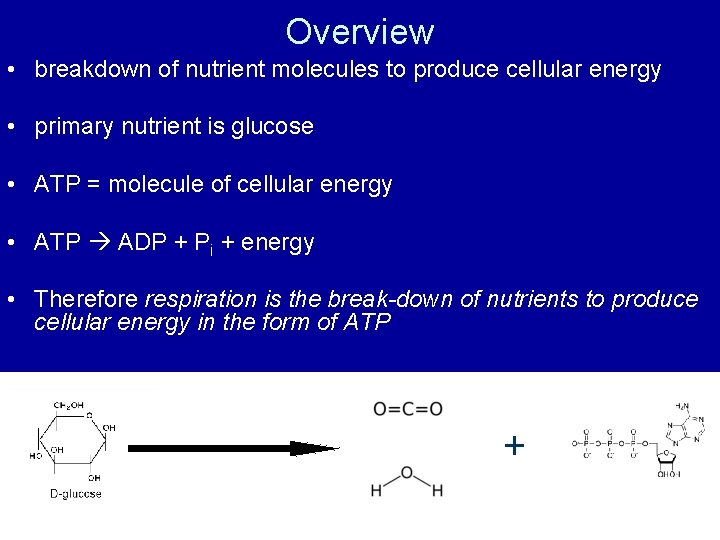
Overview • breakdown of nutrient molecules to produce cellular energy • primary nutrient is glucose • ATP = molecule of cellular energy • ATP ADP + Pi + energy • Therefore respiration is the break-down of nutrients to produce cellular energy in the form of ATP +

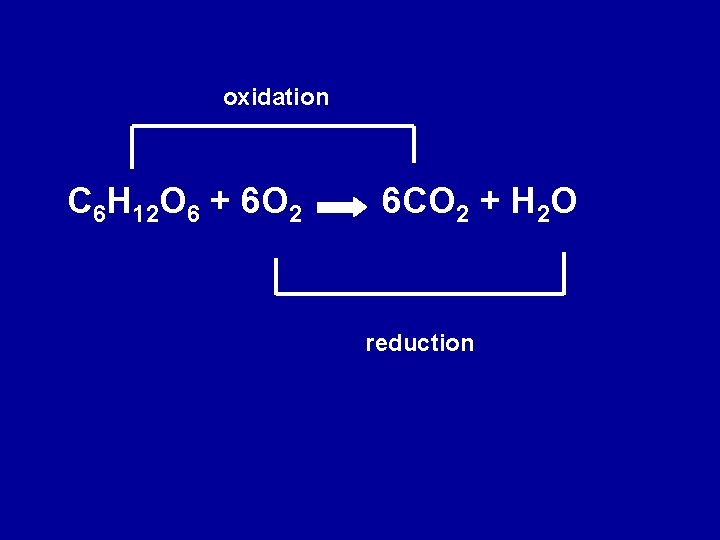
Cellular Respiration • All organisms use glucose as a primary source of energy • Oxidized to CO 2 and O 2 is reduced to water as follows: • C 6 H 1206 + 6 O 2 --> 6 CO 2 + 6 H 2 O + energy (heat and ATP) • H atoms carry electrons away from C atoms in glucose to O atoms

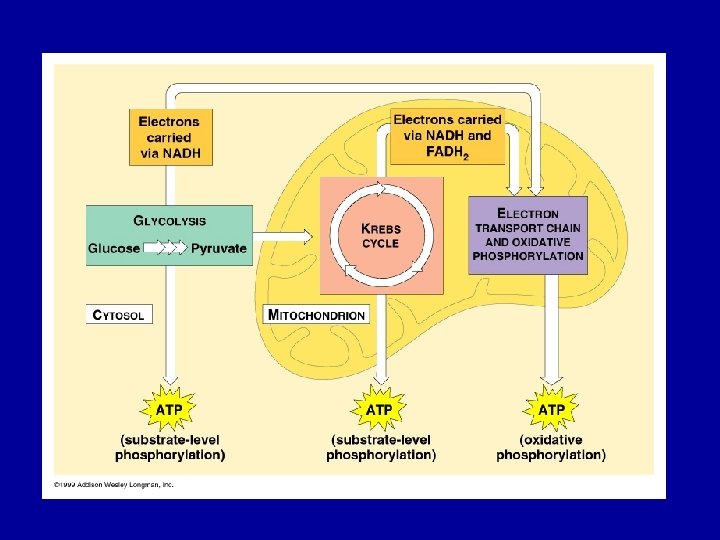
Cellular Respiration • Takes place in four steps in three different places in the cell Stage 1: Glycolysis - 10 steps, cytoplasm Stage 2: Pyruvate Oxidation - 1 step, occurring in the mitochondrial matrix Stage 3: Krebs Cycle: 8 steps, mitochondrial matrix Stage 4: Electron Transport/Chemiosmosis - multistep, mitochondrial matrix • The ultimate goal is to extract energy from nutrient molecules and store it in a form that the cell can use at any time

oxidation C 6 H 12 O 6 + 6 O 2 6 CO 2 + H 2 O reduction

• In general, organic molecules that have an abundance of H atoms are excellent food sources because they have “hilltop” electrons with the potential to “fall” closer to oxygen. • Glucose loses hydrogen atoms but they are not passed directly to oxygen. They are passed to a coenzyme first NAD+ (nicotinamide adenine dinuclotide). NAD+ serves as the oxidizing agent.

• Respiration uses an electron chain to break the “fall” of electrons to several steps. • Oxidative phosphorylation accounts for 90% of the ATP generated by respiration. • Substrate level phosphorylation produces a smaller amount of ATP. In this synthesis, ATP is produced when an enzyme transfers a phosphate from a substrate to ADP.

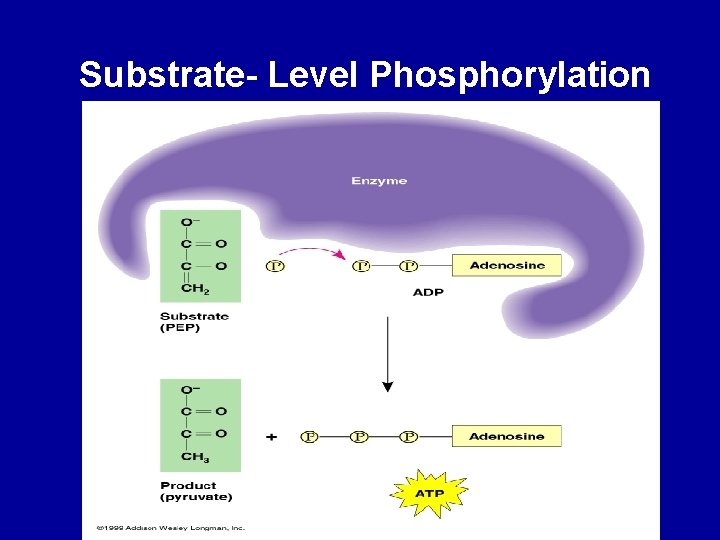
Energy Transfer Substrate Level Phosphorylation • ATP is formed directly • For each glucose molecule processed, 4 ATP molecules are generated this way in glycolysis and 2 in the Krebs cycle

Substrate- Level Phosphorylation

Oxidative Phosphorylation • ATP is formed Indirectly • Begins with Nicotinamide adenine dinucleotide (NAD+) removes 2 H atoms from the original glucose molecule • 2 e and 1 p attach to NAD+ reducing it to NADH • Flavin adenine dinucleotide (FAD) is also reduced by 2 H atoms from the original glucose to FADH 2

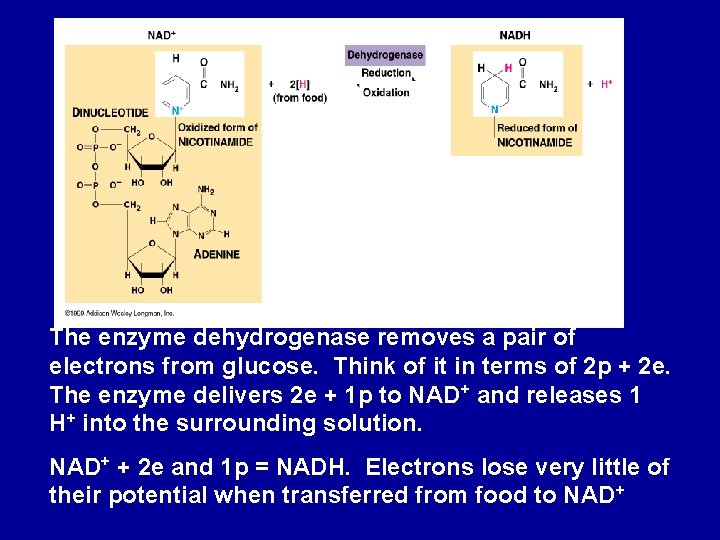
The enzyme dehydrogenase removes a pair of electrons from glucose. Think of it in terms of 2 p + 2 e. The enzyme delivers 2 e + 1 p to NAD+ and releases 1 H+ into the surrounding solution. NAD+ + 2 e and 1 p = NADH. Electrons lose very little of their potential when transferred from food to NAD+

• The breakdown of glucose is exergonic with a free energy exchange of – 686. This means that the products store less energy than the reactants. • Catabolic pathways do not directly do cellular work but are linked to work by a chemical drive shaft: ATP

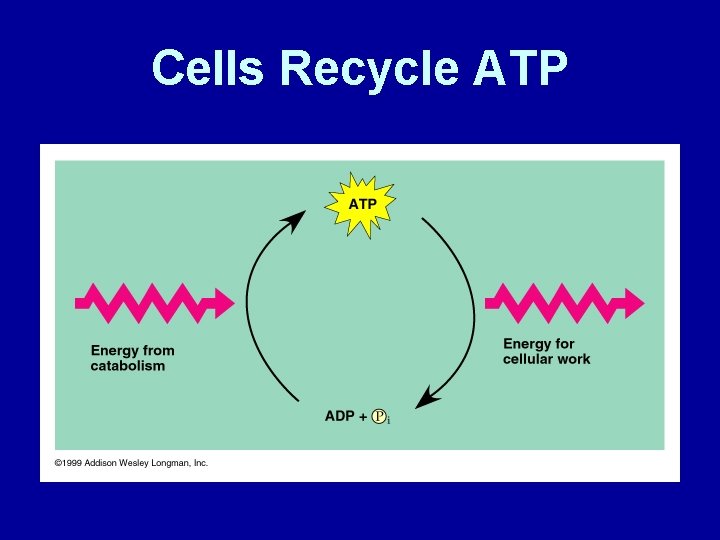
Cells Recycle ATP


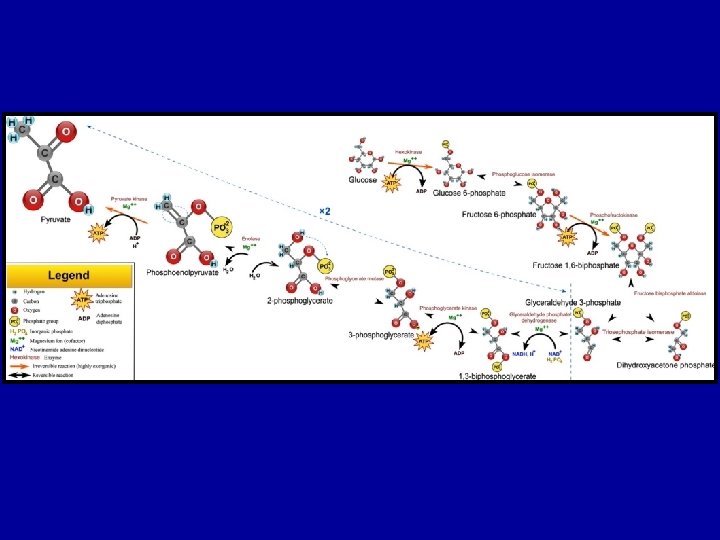
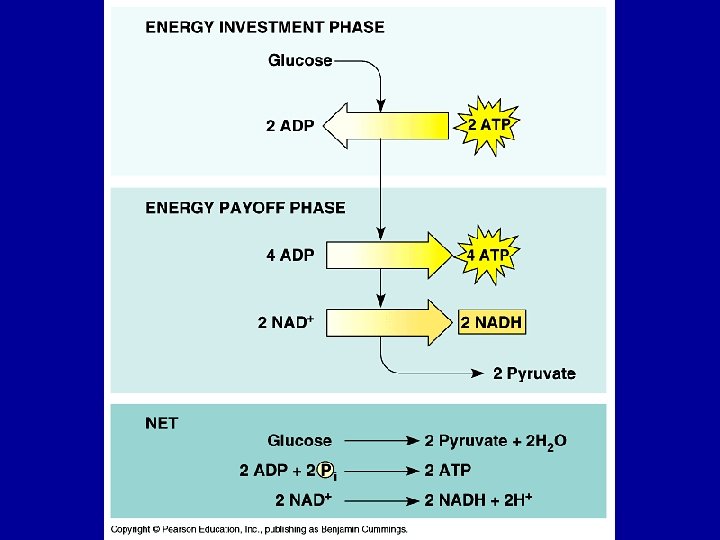
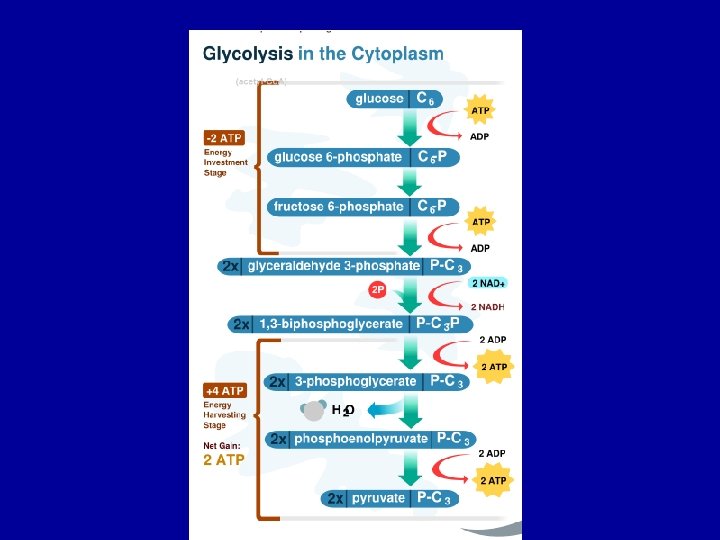
Stage 1: Glycolysis • First 10 reactions of cellular respiration • Anaerobic process • 6 C glucose is reduced to 2, 3 C pyruvate molecules • 4 ATP are produced, 2 ATP are used (2 net ATP for immediate use) • 2 NADH are produced (can be further processed to obtain more ATP)


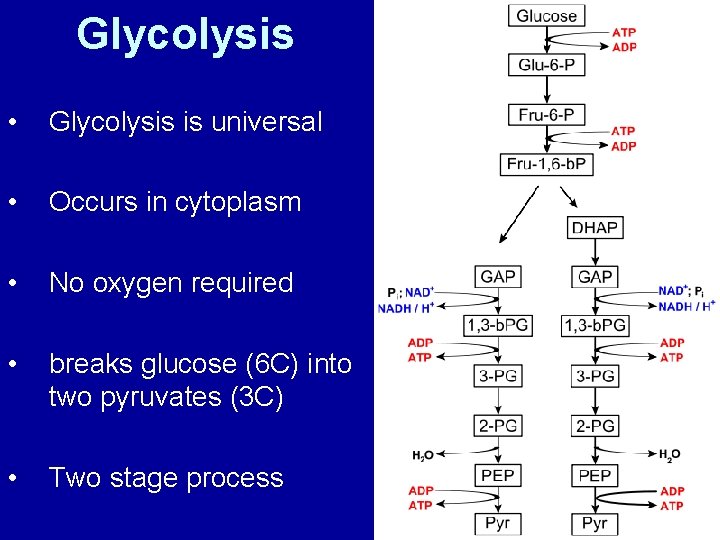
Glycolysis • Glycolysis is universal • Occurs in cytoplasm • No oxygen required • breaks glucose (6 C) into two pyruvates (3 C) • Two stage process

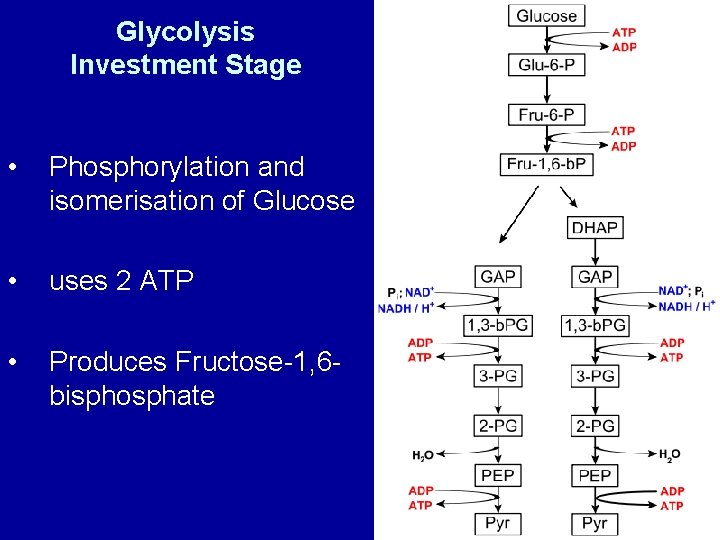
Glycolysis Investment Stage • Phosphorylation and isomerisation of Glucose • uses 2 ATP • Produces Fructose-1, 6 bisphosphate

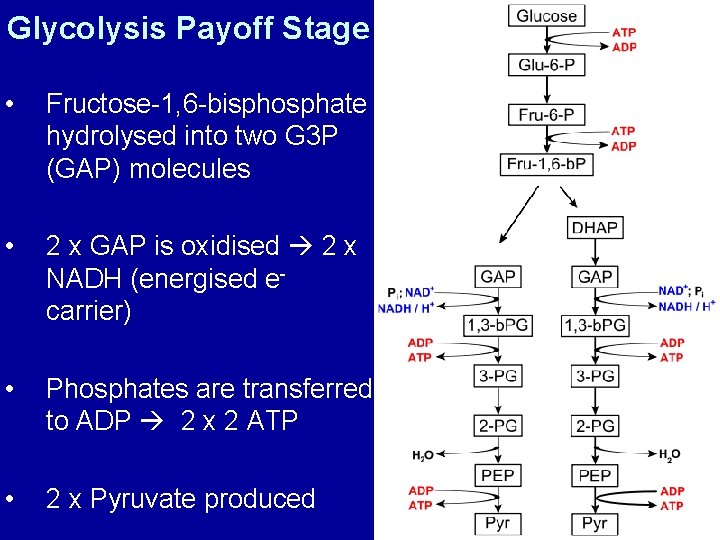
Glycolysis Payoff Stage • Fructose-1, 6 -bisphosphate hydrolysed into two G 3 P (GAP) molecules • 2 x GAP is oxidised 2 x NADH (energised e- carrier) • Phosphates are transferred to ADP 2 x 2 ATP • 2 x Pyruvate produced



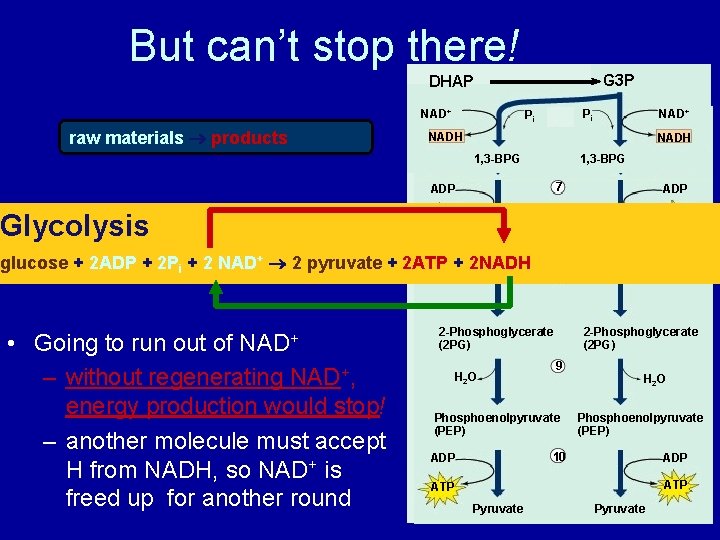
But can’t stop there! G 3 P DHAP NAD+ raw materials products Pi + NADH Pi 6 1, 3 -BPG • – without regenerating NAD+, energy production would stop! – another molecule must accept H from NADH, so NAD+ is freed up for another round Pi + NADH 7 ADP ATP 3 -Phosphoglycerate (3 PG) glucose + 2 ADP + 2 Pi + 2 NAD+ 2 pyruvate + 2 ATP + 2 NADH Going to run out of NAD+ 1, 3 -BPG ADP Glycolysis Pi 3 -Phosphoglycerate (3 PG) 8 2 -Phosphoglycerate (2 PG) H 2 O 2 -Phosphoglycerate (2 PG) 9 Phosphoenolpyruvate (PEP) H 2 O Phosphoenolpyruvate (PEP) 10 ADP ATP Pyruvate

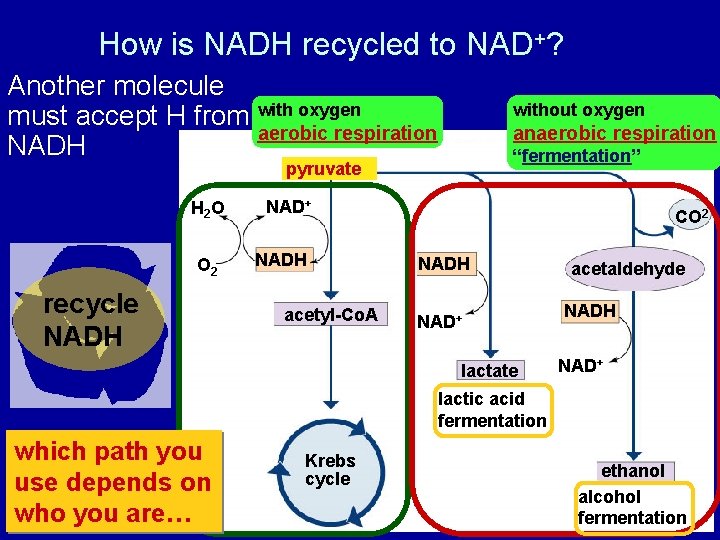
How is NADH recycled to NAD+? Another molecule must accept H from with oxygen aerobic respiration NADH without oxygen anaerobic respiration “fermentation” pyruvate H 2 O O 2 recycle NADH NAD+ NADH acetyl-Co. A CO 2 NADH NAD+ lactate acetaldehyde NADH NAD+ lactic acid fermentation which path you use depends on who you are… Krebs cycle ethanol alcohol fermentation

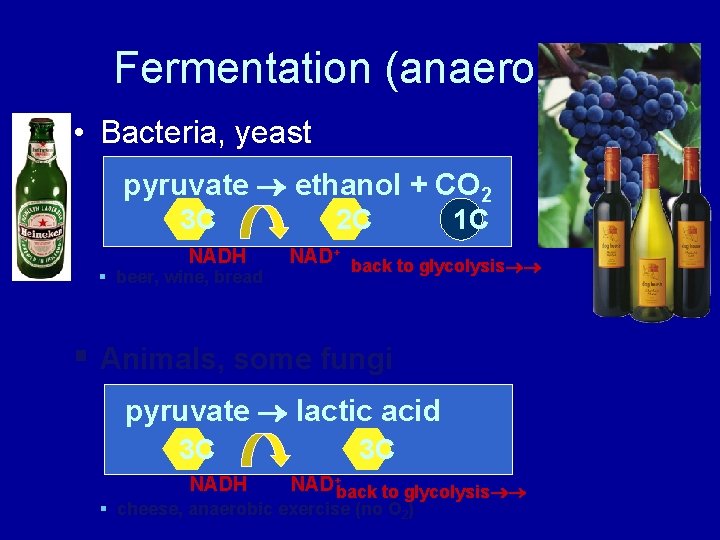
Fermentation (anaerobic) • Bacteria, yeast pyruvate ethanol + CO 2 3 C NADH § beer, wine, bread 2 C 1 C NAD+ back to glycolysis § Animals, some fungi pyruvate lactic acid 3 C NADH 3 C NAD+back to glycolysis § cheese, anaerobic exercise (no O 2)

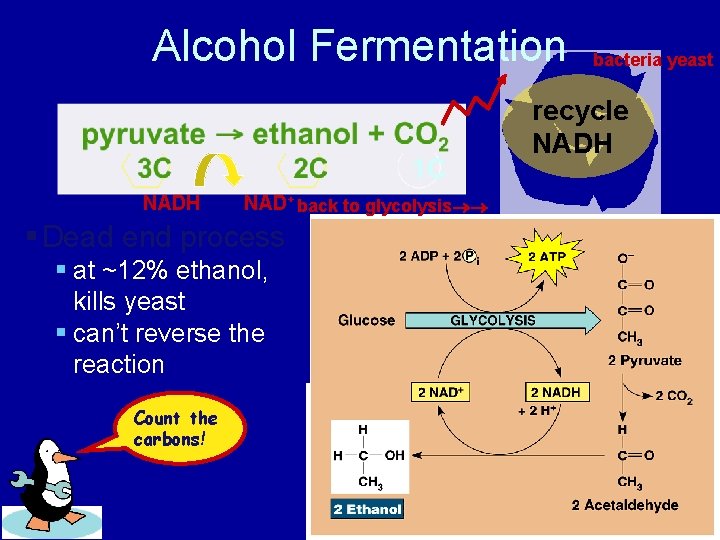
Alcohol Fermentation bacteria yeast recycle NADH NAD+ back to glycolysis § Dead end process § at ~12% ethanol, kills yeast § can’t reverse the reaction Count the carbons!

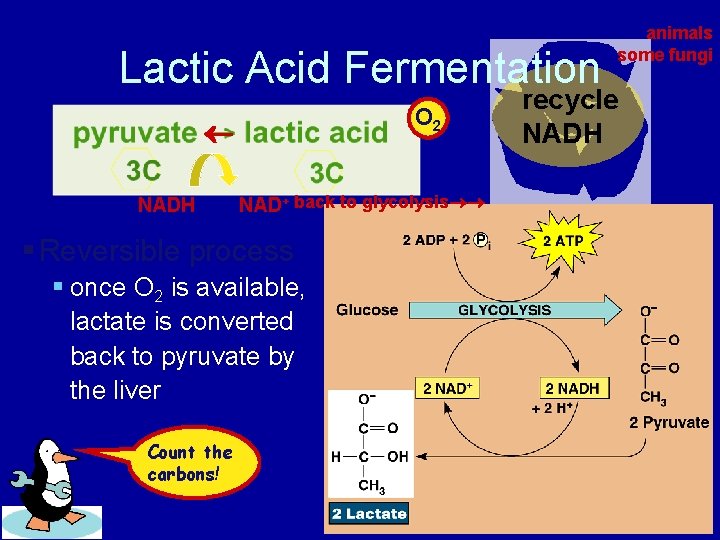
Lactic Acid Fermentation O 2 NADH NAD+ back to glycolysis § Reversible process § once O 2 is available, lactate is converted back to pyruvate by the liver Count the carbons! animals some fungi recycle NADH

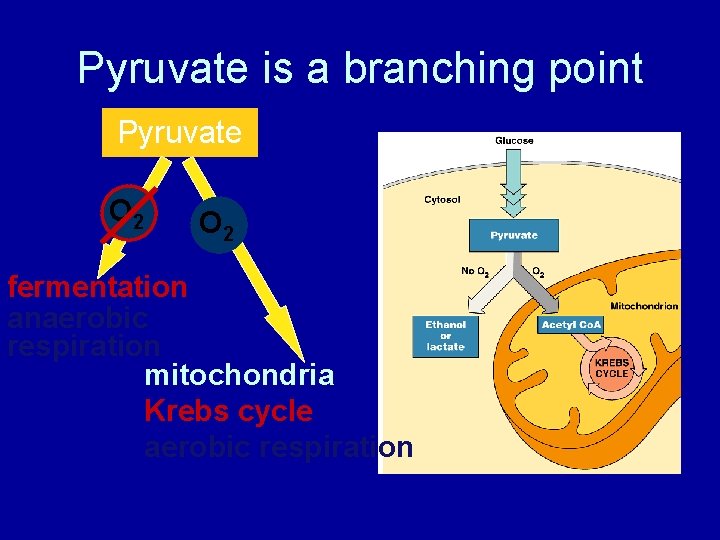
Pyruvate is a branching point Pyruvate O 2 fermentation anaerobic respiration mitochondria Krebs cycle aerobic respiration

How Efficient is Glycolysis? • Efficiency of Energy required to make ATP Glycolysis = Energy released by oxidation of glucose = 2 x 12 kcal X 100% = 3. 5% 686 kcal

Glycolysis Summary • breaks glucose (6 C) into two pyruvates (3 C) • uses 2 ATP / generates 4 ATP • net production of 2 ATP per glucose • generates 2 NADH molecules

Concept Check 1. 2. 3. 4. Where does glycolysis occur? What is the oxidizing agent of glycolysis? How many ATP’s are invested in glycolysis? By the end of glycolysis how many of the following are produced? a. Net ATP’s b. NADH’s c. Pyruvates 5. What has more energy 2 G 3 P’s or 1 glucose? 6. Where is most of the energy by the end of glycolysis?

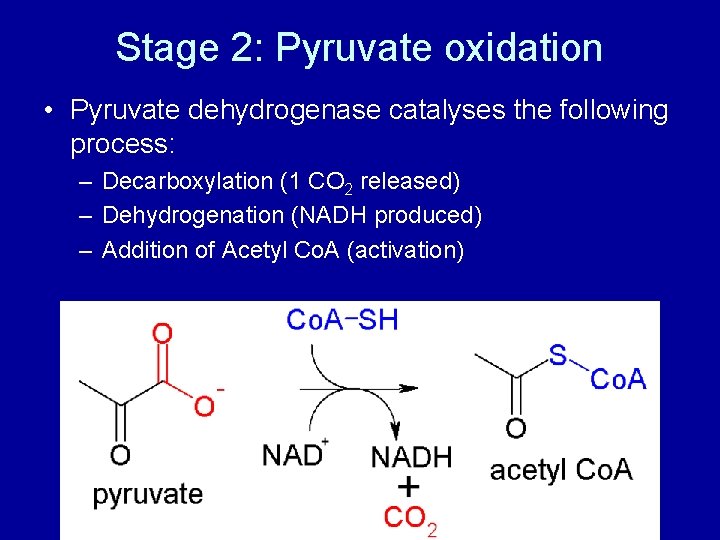
Stage 2: Pyruvate oxidation • Pyruvate dehydrogenase catalyses the following process: – Decarboxylation (1 CO 2 released) – Dehydrogenation (NADH produced) – Addition of Acetyl Co. A (activation)

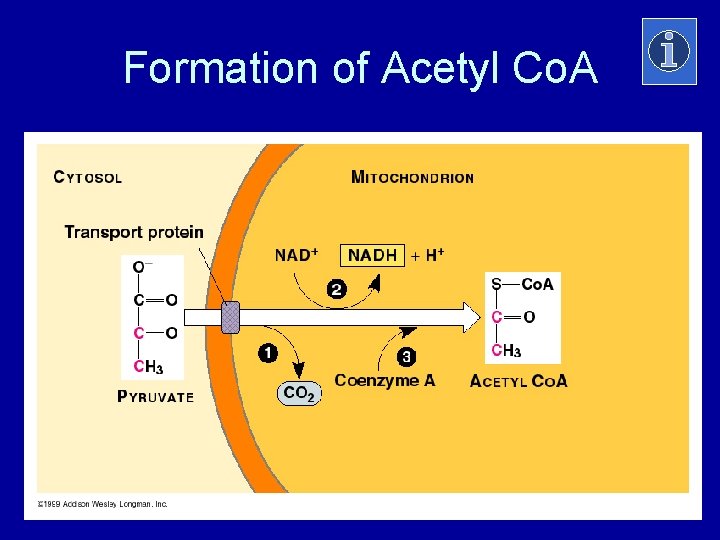
Formation of Acetyl Co. A

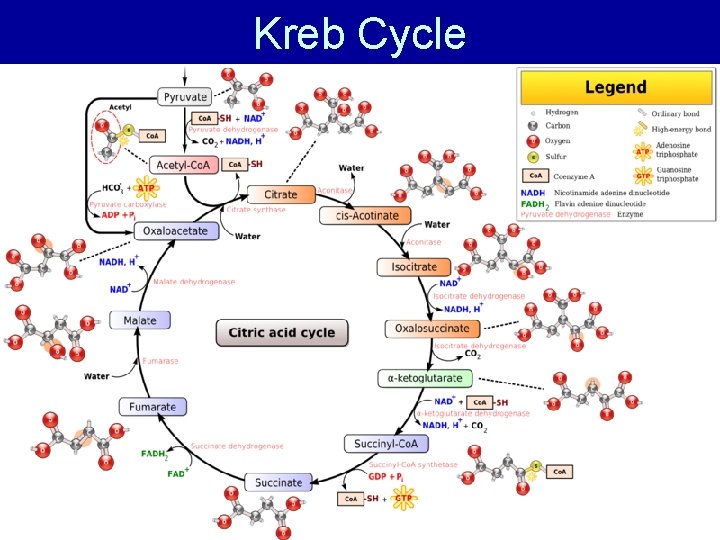
Stage 3: Kreb’s Cycle • A. k. a. Citric Acid Cycle • occurs in the mitochondrial matrix => pyruvate diffuses into the mitochondrial matrix • Aerobic (requires oxygen) • Pyruvate is completely oxidised to CO 2 • Starts with pyruvate oxidation followed by Acetly Co. A oxidation

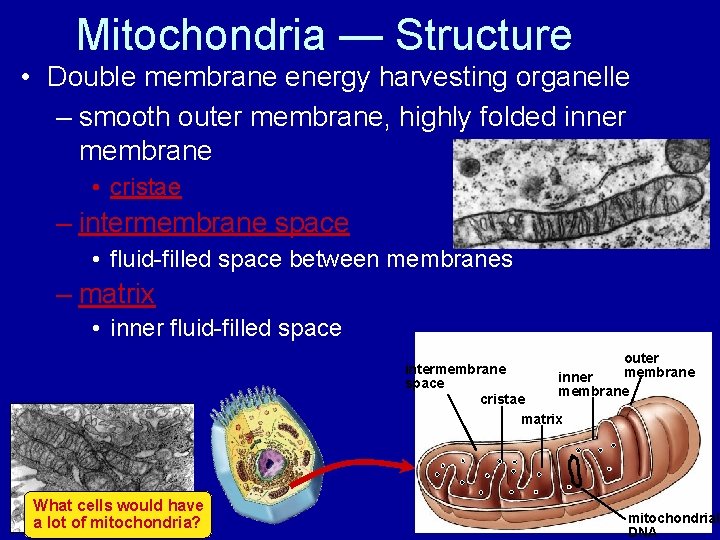
Mitochondria — Structure • Double membrane energy harvesting organelle – smooth outer membrane, highly folded inner membrane • cristae – intermembrane space • fluid-filled space between membranes – matrix • inner fluid-filled space outer intermembrane inner space membrane cristae matrix What cells would have a lot of mitochondria? mitochondrial DNA

Kreb Cycle

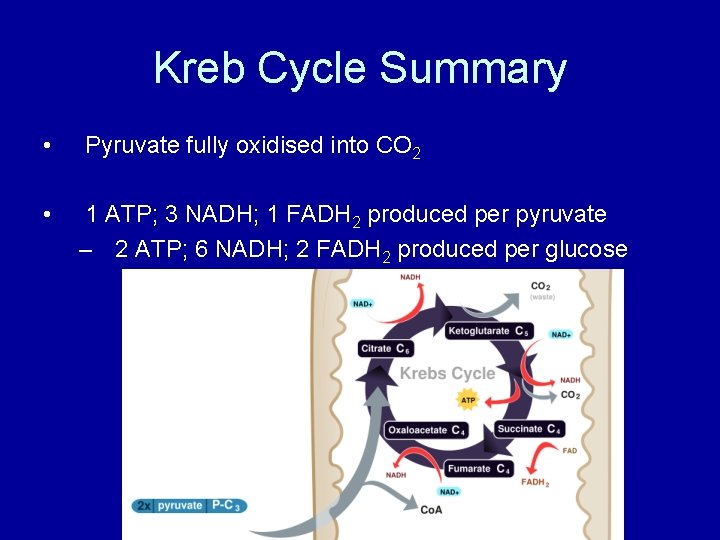
Kreb Cycle Summary • Pyruvate fully oxidised into CO 2 • 1 ATP; 3 NADH; 1 FADH 2 produced per pyruvate – 2 ATP; 6 NADH; 2 FADH 2 produced per glucose

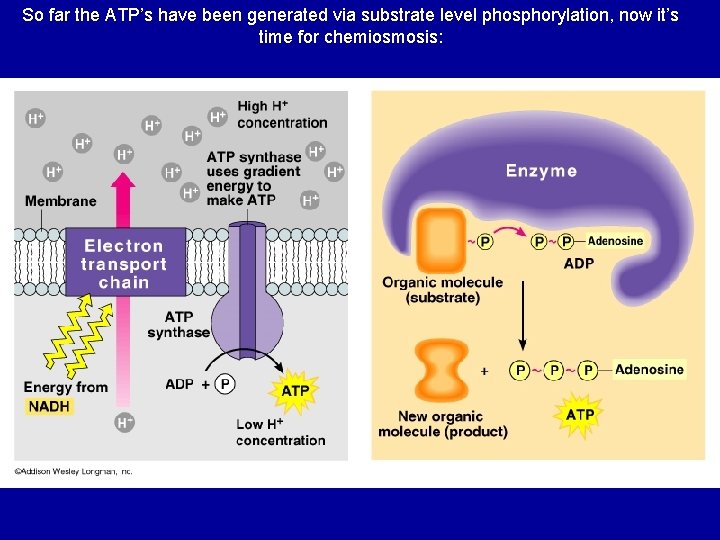
So far the ATP’s have been generated via substrate level phosphorylation, now it’s time for chemiosmosis:

Energy Carriers Both NADH and FADH 2 that were reduced during either glycolysis or the Kreb’s Cycle have a lot of energy stored within them. The energy is stored in the electrons they carry. Produced in the mitochondria: 1 molecule of NADH = 3 ATP 1 molecule of FADH 2 = 2 ATP Produced in the cytoplasm (glycolysis): 1 molecule of NADH = 2 ATP there is an energy cost to transporting NADH across mitochondrial membrane (this depends on how they get across, does not always cost energy)

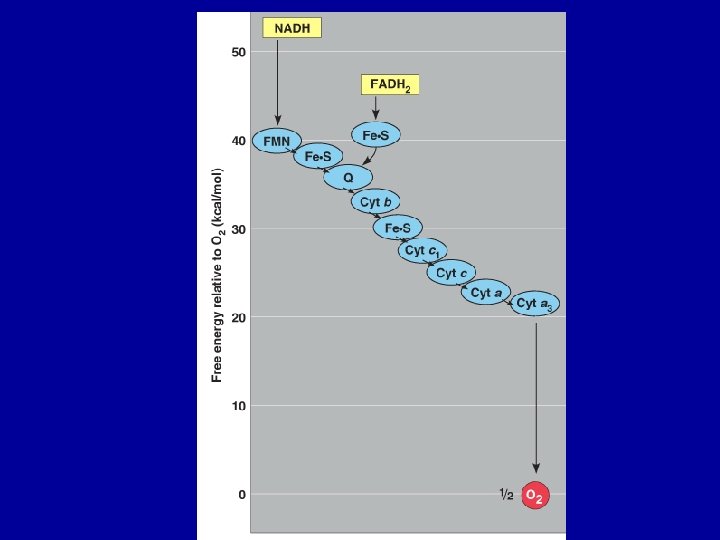
Stage 4: The Electron Transport Chain • • • Regenerates electron carriers uses a series of enzymes on the inner mitochondrial membrane It is a series of electron carrier molecules, that transfer electrons to each other through a connected series of redox reactions. Energy from electrons transfer into H+ gradient drives ATP synthesis (~34 ATP per glucose) OXIDATIVE PHOSPHORYLATION electrons finally “sunk” into water production


What is the role of oxygen? • The highly electronegative oxygen is the only molecule capable of receiving the stable electron from cytochrome a 3. • The cytochrome electron carriers are proteins with heme prosthetic group. • The iron of the cytochromes transfers electrons • The oxygen uses the electrons to make water

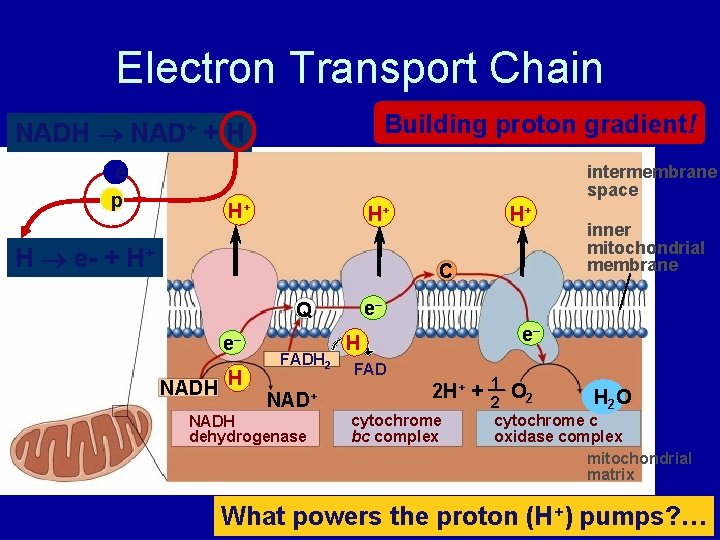
Electron Transport Chain Building proton gradient! NADH NAD+ + H e p intermembrane space H+ H+ H e- + H+ H+ C e– Q e– NADH H FADH 2 NAD+ NADH dehydrogenase inner mitochondrial membrane e– H FAD 2 H+ + cytochrome bc complex 1 O 2 H 2 O 2 cytochrome c oxidase complex mitochondrial matrix What powers the proton (H+) pumps? …

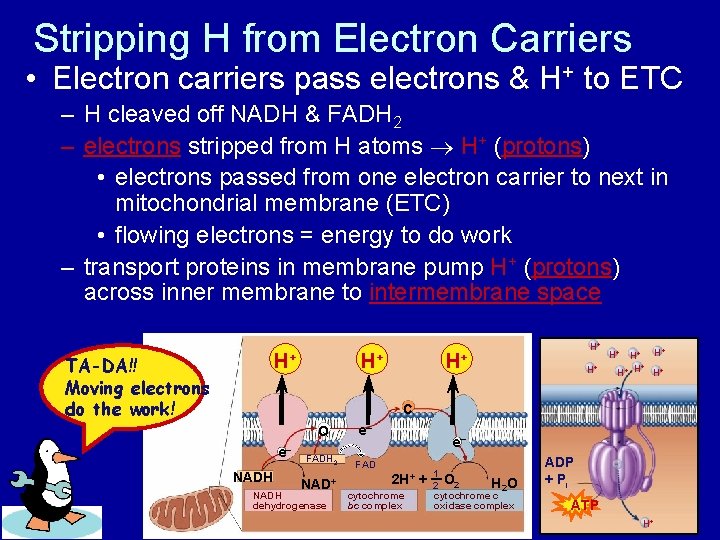
Stripping H from Electron Carriers • Electron carriers pass electrons & H+ to ETC – H cleaved off NADH & FADH 2 – electrons stripped from H atoms H+ (protons) • electrons passed from one electron carrier to next in mitochondrial membrane (ETC) • flowing electrons = energy to do work – transport proteins in membrane pump H+ (protons) across inner membrane to intermembrane space + H H+ TA-DA!! Moving electrons do the work! + H H+ H+ H+ H H+ C e– NADH Q e– FADH 2 FAD NAD+ NADH dehydrogenase e– 2 H+ cytochrome bc complex + 1 H 2 O 2 cytochrome c oxidase complex ADP + Pi ATP H+

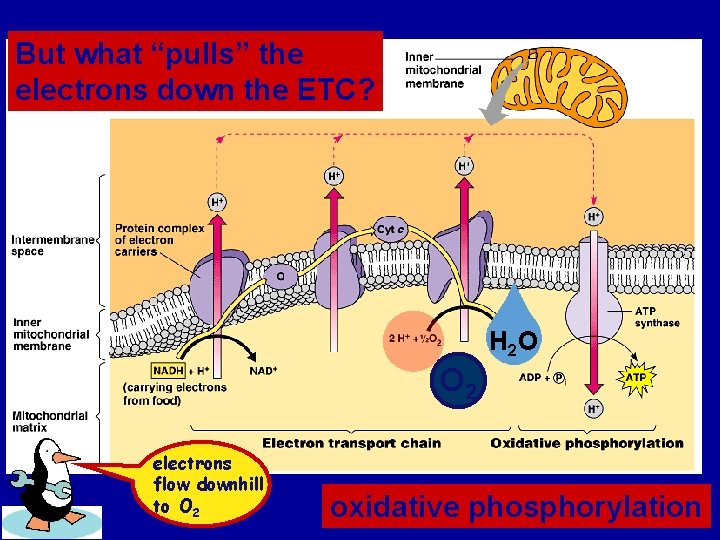
But what “pulls” the electrons down the ETC? H 2 O O 2 electrons flow downhill to O 2 oxidative phosphorylation

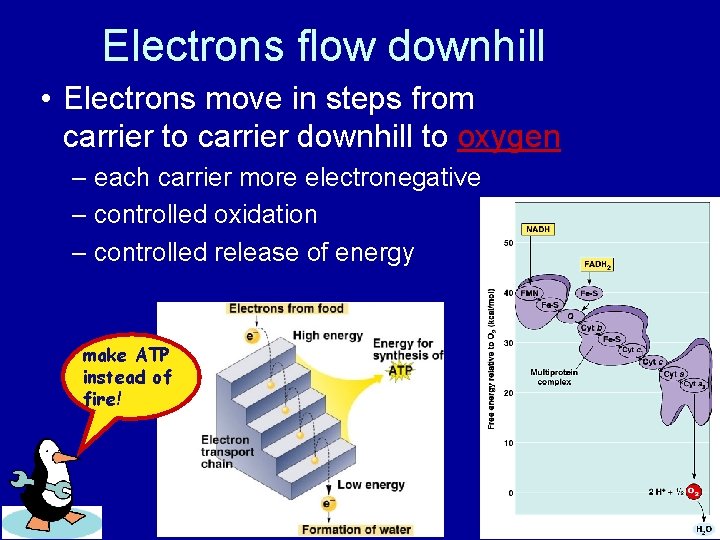
Electrons flow downhill • Electrons move in steps from carrier to carrier downhill to oxygen – each carrier more electronegative – controlled oxidation – controlled release of energy make ATP instead of fire!

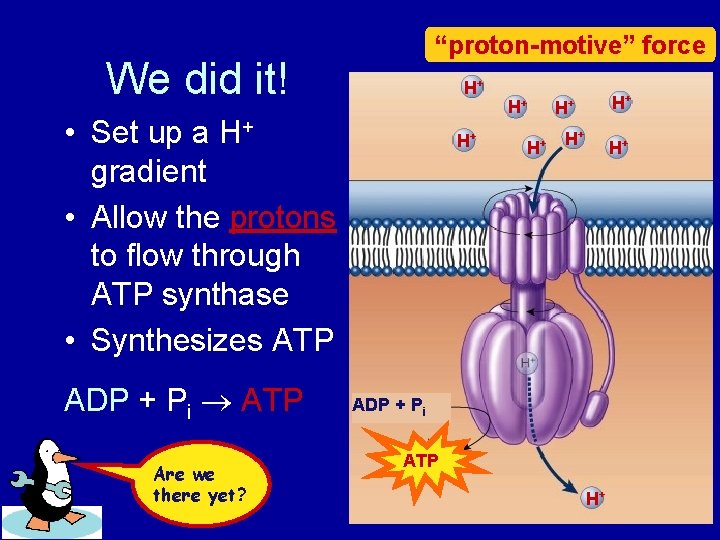
“proton-motive” force We did it! H+ • Set up a H+ gradient • Allow the protons to flow through ATP synthase • Synthesizes ATP ADP + Pi ATP Are we there yet? H+ H+ ADP + Pi ATP H+

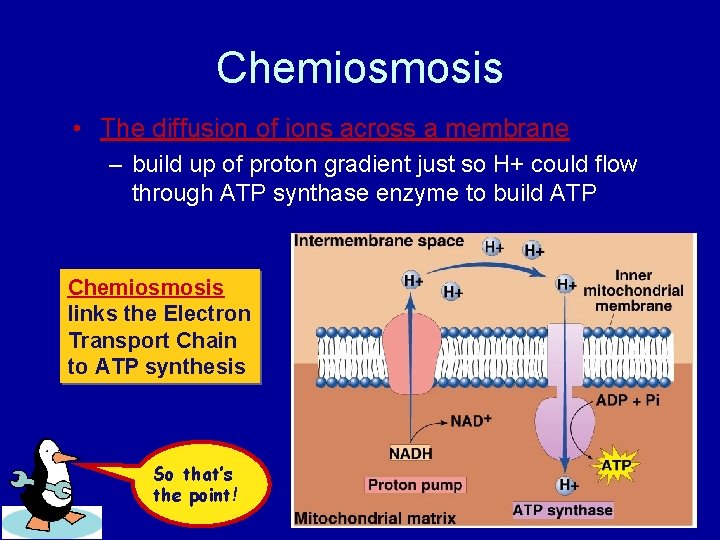
Chemiosmosis • The diffusion of ions across a membrane – build up of proton gradient just so H+ could flow through ATP synthase enzyme to build ATP Chemiosmosis links the Electron Transport Chain to ATP synthesis So that’s the point!

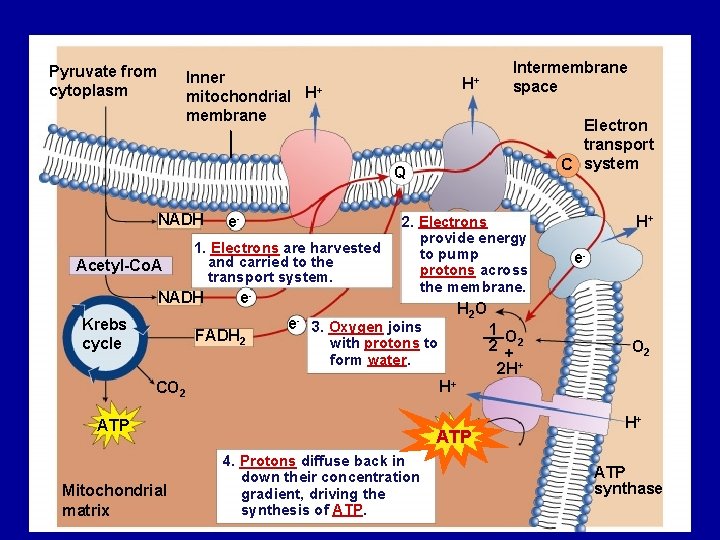
Pyruvate from cytoplasm Inner + mitochondrial H membrane H+ Intermembrane space Electron transport C system Q NADH Acetyl-Co. A 1. Electrons are harvested and carried to the transport system. NADH Krebs cycle e- e- FADH 2 e- 2. Electrons provide energy to pump protons across the membrane. e- H 2 O 3. Oxygen joins with protons to form water. 1 O 2 +2 2 H+ O 2 H+ CO 2 ATP Mitochondrial matrix H+ ATP 4. Protons diffuse back in down their concentration gradient, driving the synthesis of ATP. H+ ATP synthase

• 2 NADH’s from glycolysis ---->6 ATP’s • 2 NADH’s from transition ----->6 ATP’s • 6 NADH’s from the mitochondrial matrix ---> 18 ATP’s • 2 FADH 2’s from KC -----> 4 ATP’s • Total ATP’s from oxidative phosphorylation = 34 ATP’s • ATP’s from substrate level posphorylation = 4 • GRAND TOTAL = 36 - 38 ATP’s

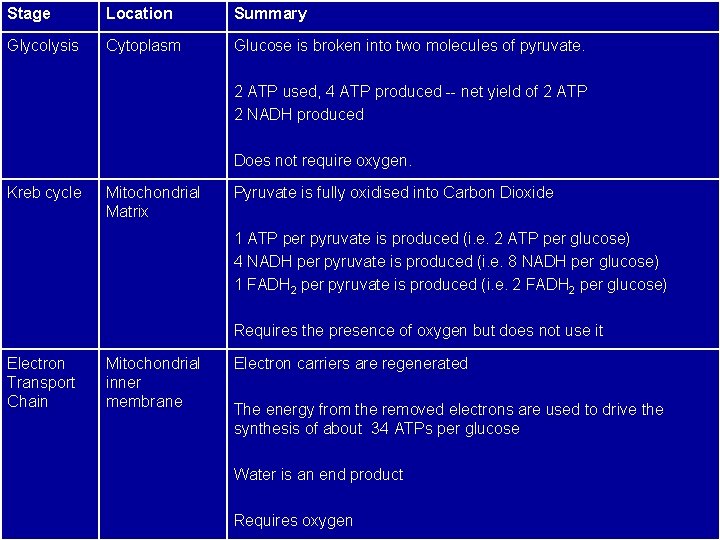
Stage Location Summary Glycolysis Cytoplasm Glucose is broken into two molecules of pyruvate. 2 ATP used, 4 ATP produced -- net yield of 2 ATP 2 NADH produced Does not require oxygen. Kreb cycle Mitochondrial Matrix Pyruvate is fully oxidised into Carbon Dioxide 1 ATP per pyruvate is produced (i. e. 2 ATP per glucose) 4 NADH per pyruvate is produced (i. e. 8 NADH per glucose) 1 FADH 2 per pyruvate is produced (i. e. 2 FADH 2 per glucose) Requires the presence of oxygen but does not use it Electron Transport Chain Mitochondrial inner membrane Electron carriers are regenerated The energy from the removed electrons are used to drive the synthesis of about 34 ATPs per glucose Water is an end product Requires oxygen

Review Questions

1. Glucose, made from six radioactively labeled carbon atoms, is fed to yeast cells in the absence of oxygen. How many molecules of radioactive alcohol (C 2 H 5 OH) are formed from each molecule of glucose? A. B. C. D. E. 0 1 2 3 6

2. Which kind of metabolic poison would most directly interfere with glycolysis? A. an agent that reacts with oxygen and depletes its concentration in the cell B. an agent that binds to pyruvate and inactivates it C. an agent that closely mimics the structure of glucose but is not metabolized D. an agent that reacts with NADH and oxidizes it to NAD+ E. an agent that inhibits the formation of acetyl coenzyme A

3. Which of the following does not occur in glycolysis? A. Phosphorylation B. Oxidation C. ATP Consumption D. Enzyme-catalyzed catabolism E. Catabolism of pyruvate

4. Why would an anaerobically respiring cell not be able to continue glycolysis if it did not carry out fermentation?

5. During cellular respiration, the compound acetyl-Co. A is A. Converted from acetic acid within the electron transport chain. B. Converted from carbon dioxide during anaerobic conditions C. Necessary for the initiation of glycolysis D. Converted from pyruvate in the presence of oxygen E. Necessary for the splitting of water.

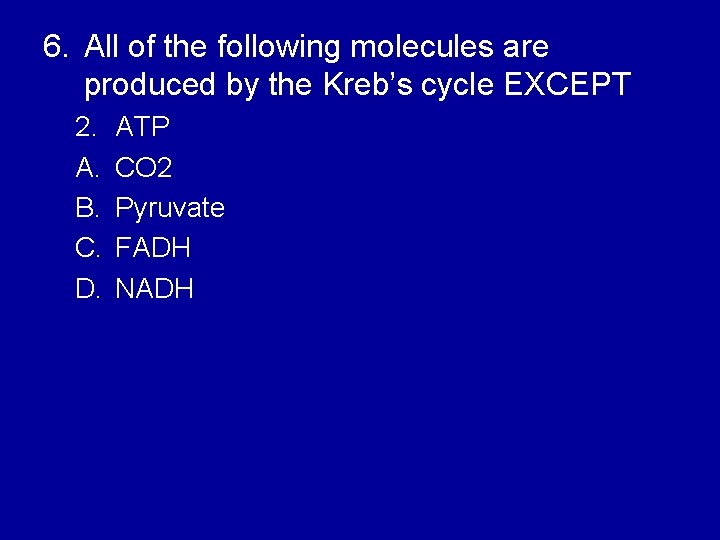
6. All of the following molecules are produced by the Kreb’s cycle EXCEPT 2. A. B. C. D. ATP CO 2 Pyruvate FADH NADH

7. By the end of the Kreb’s cycle, which of the following statements are true I. All of the carbon from the original glucose molecule has been oxidized to Carbon Dioxide II. All of the electrons from the original glucose molecule have been transferred to ATP molecules III. Oxygen is no longer necessary for the remaining steps of aerobic respiration A. B. C. D. E. I only III only I and II only I, and III only

8. Diagram Time! 1. Beginning with a Glucose molecule, go through glycolysis. Then a fork in the road: Fermentation vs. The Citric Acid Cycle. 2. Keep Track of the following: • Starting compounds, ending compounds (indicate names and # of carbons). • At the end of each step, show much ATP, NADH and FADH 2 is produced. • Explain the fates of all materials you have at the end.

Review Questions

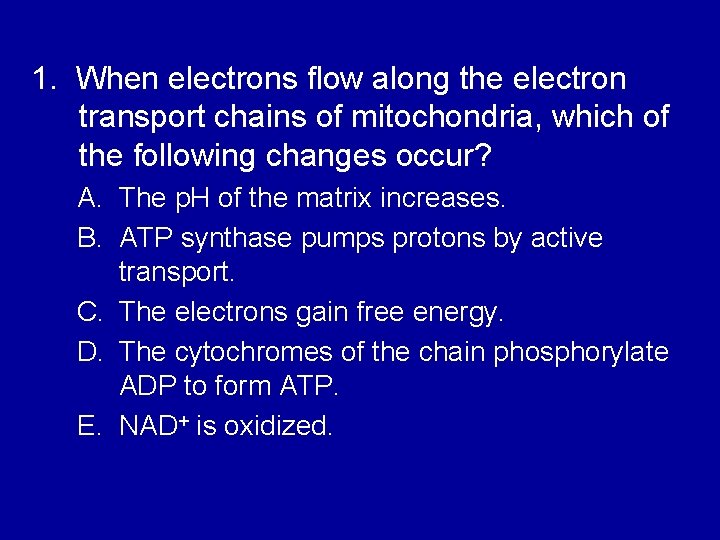
1. When electrons flow along the electron transport chains of mitochondria, which of the following changes occur? A. The p. H of the matrix increases. B. ATP synthase pumps protons by active transport. C. The electrons gain free energy. D. The cytochromes of the chain phosphorylate ADP to form ATP. E. NAD+ is oxidized.

2. In the presence of a metabolic poison that specifically and completely inhibit the function of mitochondrial ATP synthase, which of the following would you expect? A. a decrease in the p. H difference across the inner mitochondrial membrane B. an increase in the p. H difference across the inner mitochondrial membrane C. increased synthesis of ATP D. oxygen consumption to cease E. proton pumping by the electron transport chain to cease

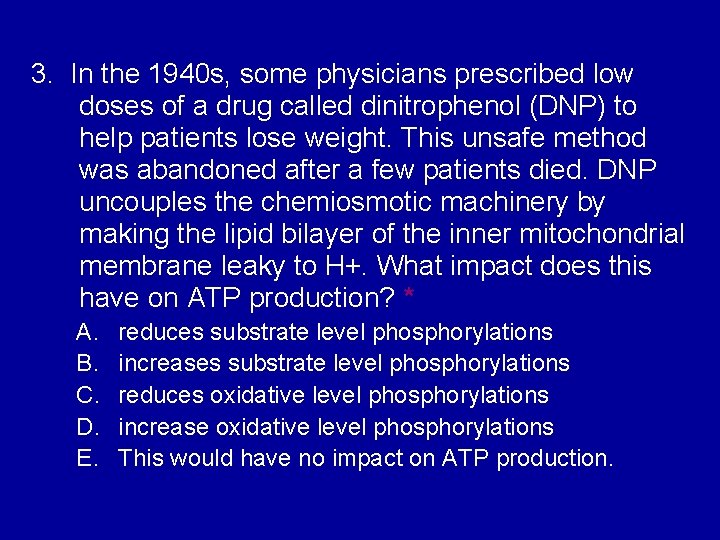
3. In the 1940 s, some physicians prescribed low doses of a drug called dinitrophenol (DNP) to help patients lose weight. This unsafe method was abandoned after a few patients died. DNP uncouples the chemiosmotic machinery by making the lipid bilayer of the inner mitochondrial membrane leaky to H+. What impact does this have on ATP production? * A. B. C. D. E. reduces substrate level phosphorylations increases substrate level phosphorylations reduces oxidative level phosphorylations increase oxidative level phosphorylations This would have no impact on ATP production.

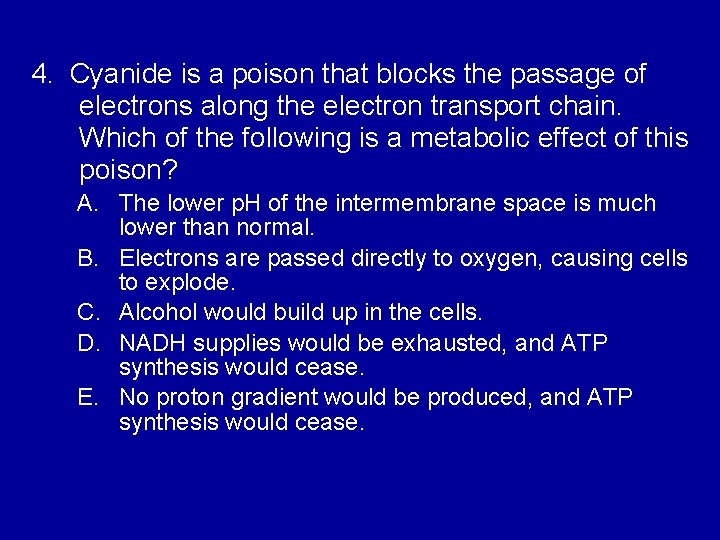
4. Cyanide is a poison that blocks the passage of electrons along the electron transport chain. Which of the following is a metabolic effect of this poison? A. The lower p. H of the intermembrane space is much lower than normal. B. Electrons are passed directly to oxygen, causing cells to explode. C. Alcohol would build up in the cells. D. NADH supplies would be exhausted, and ATP synthesis would cease. E. No proton gradient would be produced, and ATP synthesis would cease.

5. A young relative of yours has never had much energy. He goes to a doctor for help and is sent to the hospital for some tests. There they discover his mitochondria can use only fatty acids and amino acids for respiration, and his cells produce more lactate than normal. Of the following, which is the best explanation of his condition? A. B. C. D. E. His mitochondria lack the transport protein that moves pyruvate across the outer mitochondrial membrane. His cells can not move NADH from glycolysis into the mitochondria. His cells contain something that inhibits oxygen use in his mitochondria. His cells lack the enzyme in glycolysis that forms pyruvate. His cells have a defective electron transport chain, so glucose goes to lactate instead of to acetyl Co. A.

6. You have a friend who lost 15 pounds of fat on a diet. Where did the fat go (how was it lost)? * A. It was released as CO 2 and H 2 O. B. Chemical energy was converted to heat and then released. C. It was converted to ATP, which weighs much less than fat. D. It was broken down to amino acids and eliminated from the body. E. It was converted to urine and eliminated from the body.

7. Diagram Time! Draw a symbolic representation of the mitochondrial electron transport chain (include the matrix and the inter-membrane space). Show the interactions of NADH, FADH 2, electrons, protons, ADP, Pi, and ATP with the electron transport chain that lead to oxidative phosphorylation.

You Would Like a Rap Song?