Cellular Respiration How do cells extract energy from




- Slides: 63

Cellular Respiration How do cells extract energy from glucose?

Cellular Respiration Where did Chuck Norris get all that energy from?

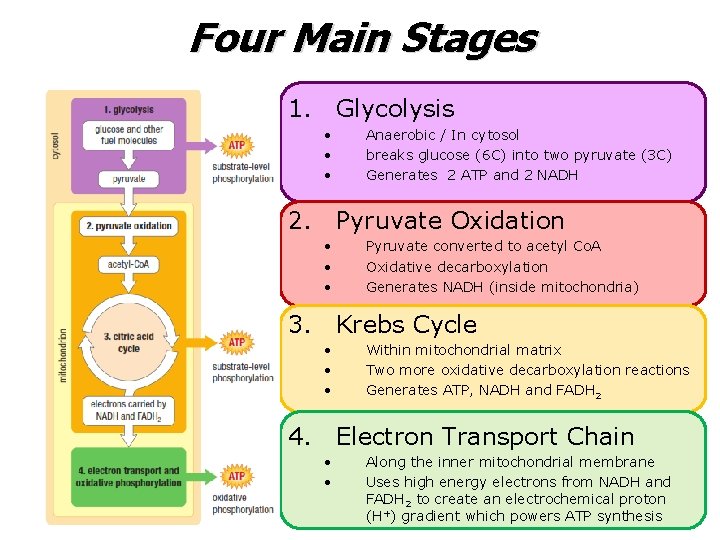
What is Cellular Respiration? • Cellular respiration – An aerobic process (requires oxygen) O 2 – Uses chemical energy from glucose to make ATP 1 cellular resp. glucose 36 ATP – The chemical energy stored in ATP can be used throughout the cell

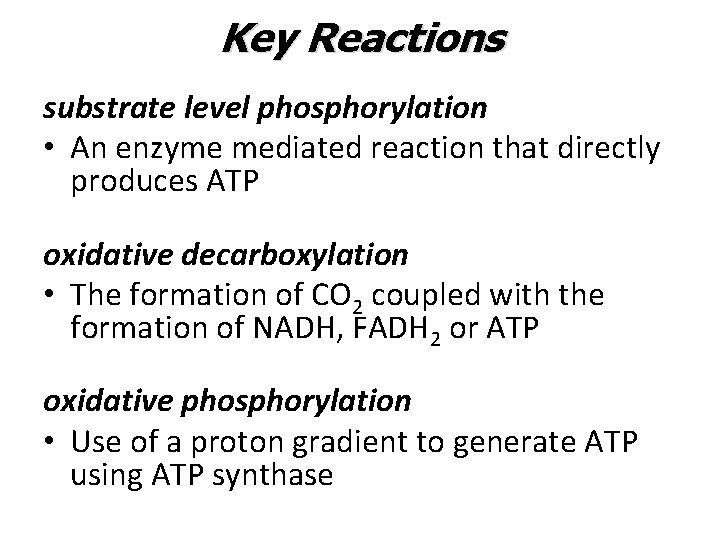
Four Main Stages 1. Glycolysis • • • Anaerobic / In cytosol breaks glucose (6 C) into two pyruvate (3 C) Generates 2 ATP and 2 NADH 2. Pyruvate Oxidation • • • Pyruvate converted to acetyl Co. A Oxidative decarboxylation Generates NADH (inside mitochondria) 3. Krebs Cycle • • • Within mitochondrial matrix Two more oxidative decarboxylation reactions Generates ATP, NADH and FADH 2 4. Electron Transport Chain • • Along the inner mitochondrial membrane Uses high energy electrons from NADH and FADH 2 to create an electrochemical proton (H+) gradient which powers ATP synthesis

Key Reactions substrate level phosphorylation • An enzyme mediated reaction that directly produces ATP oxidative decarboxylation • The formation of CO 2 coupled with the formation of NADH, FADH 2 or ATP oxidative phosphorylation • Use of a proton gradient to generate ATP using ATP synthase

Do Cells NEED Mitochondria? • It is possible to generate relatively small amounts of ATP relying exclusively on the reactions in the cytoplasm • Fermentation is essentially glycolysis, however, the final products are slightly modified • We will return to this process in detail after learning about cellular respiration

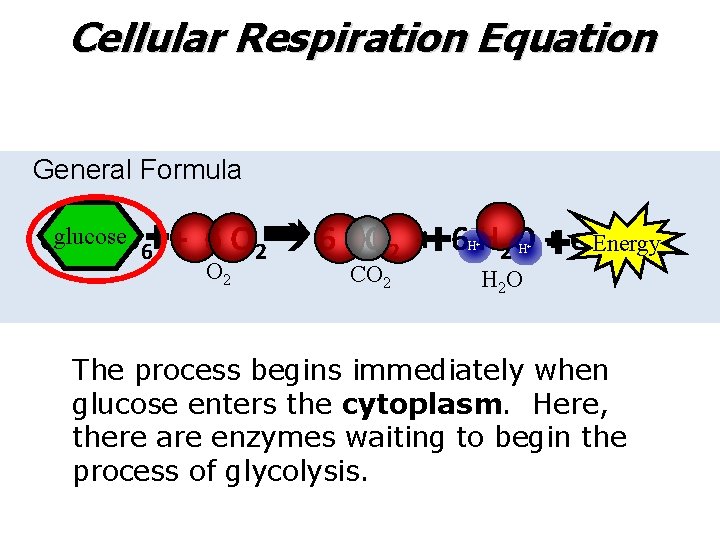
Cellular Respiration Equation General Formula Cglucose Energy 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + energy H+ O 2 CO 2 H+ H 2 O The process begins immediately when glucose enters the cytoplasm. Here, there are enzymes waiting to begin the process of glycolysis.

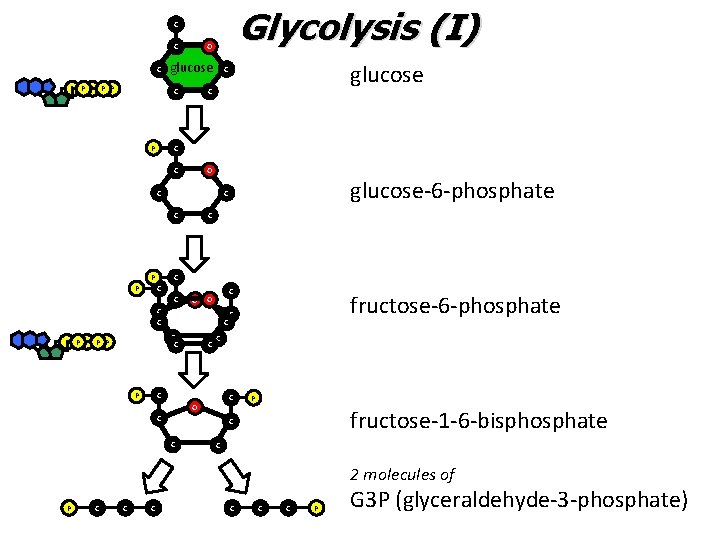
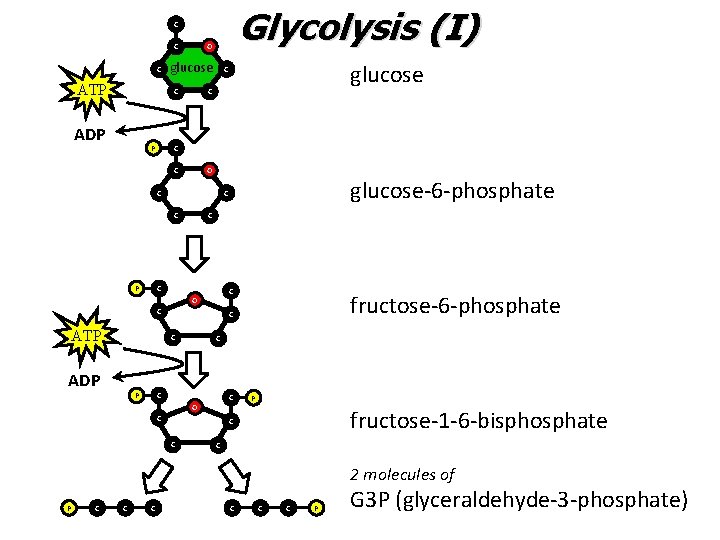
Glycolysis I • The “investment” period – ATP is USED to activate glucose – This is accomplished via phosphorylation reactions • Adding a phosphate group from ATP

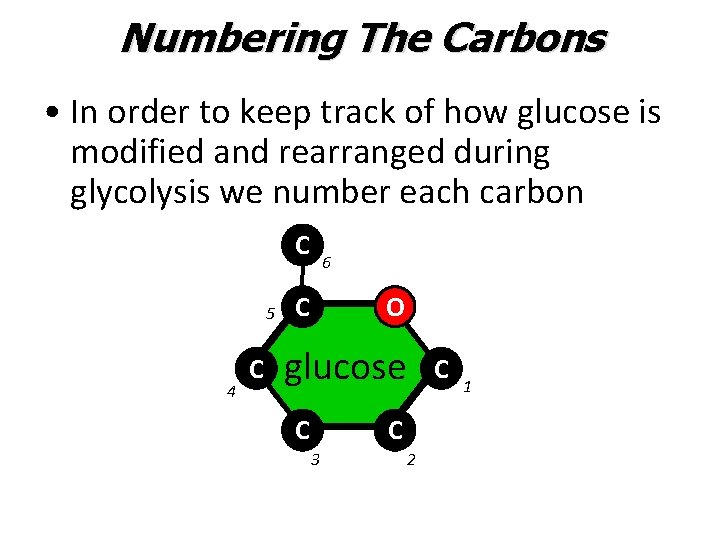
Numbering The Carbons • In order to keep track of how glucose is modified and rearranged during glycolysis we number each carbon C 5 4 C 6 C O glucose C C 3 2 C 1

Glycolysis (I) C C C P P P O glucose C P C C C O C P C C O C C C P P P glucose-6 -phosphate C C P glucose C C fructose-6 -phosphate C C O C P fructose-1 -6 -bisphosphate C C C 2 molecules of P C C C P G 3 P (glyceraldehyde-3 -phosphate)

Glycolysis (I) C C C ATP O glucose C ADP P C C C O C ATP P fructose-6 -phosphate C C ADP glucose-6 -phosphate C C P glucose C C O C P fructose-1 -6 -bisphosphate C C C 2 molecules of P C C C P G 3 P (glyceraldehyde-3 -phosphate)

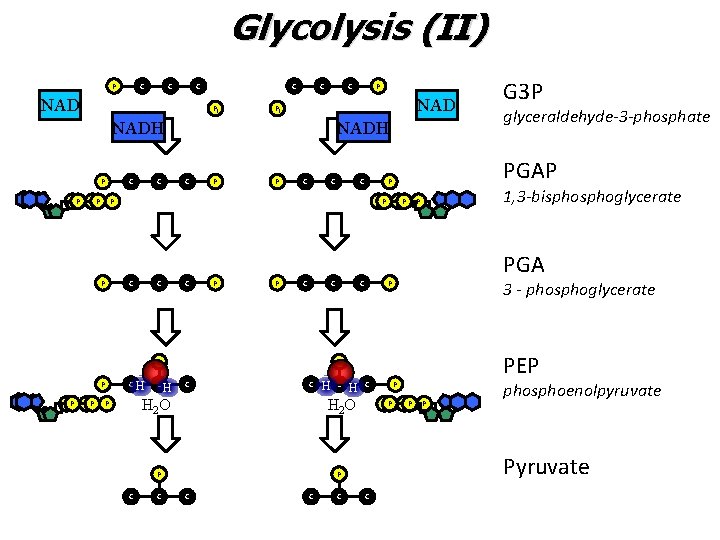
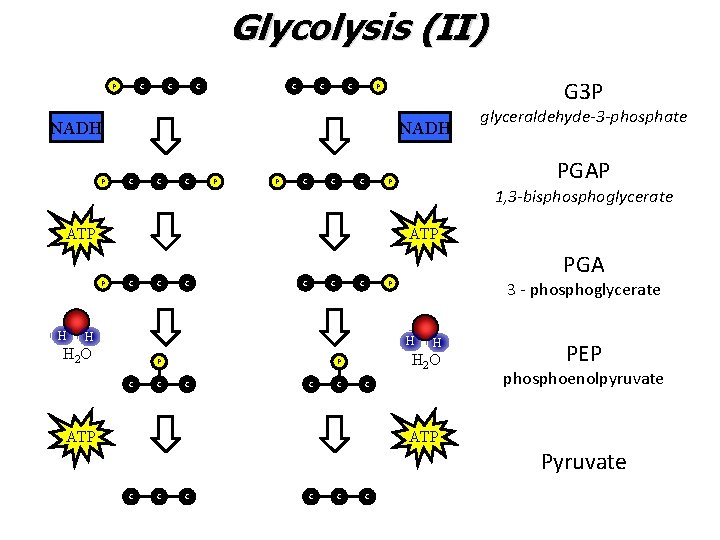
Glycolysis (II) • The “pay-off” period ‒ ATP and NADH (a temporary high energy carrier) are PRODUCED during glycolysis II ‒ By the end of glycolysis II, glucose has been broken down to two 3 carbon compounds called pyruvate (pyruvic acid)

Glycolysis (II) P C C C NAD C Pi C C NAD Pi NADH P PP PP C C NADH C P P C C P C H C C C H C PGA 3 - phosphoglycerate PEP C C PP P phosphoenolpyruvate P C P PP glyceraldehyde-3 -phosphate 1, 3 -bisphoglycerate P P H 2 O P C PP P H 2 O P C G 3 P PGAP P PP C P P PP P C

Glycolysis (II) P C C C G 3 P P NADH C C C P P C C C 1, 3 -bisphoglycerate ATP P H PGAP P ATP glyceraldehyde-3 -phosphate C C C PGA P H 3 - phosphoglycerate H H 2 O P C C C H C ATP PEP phosphoenolpyruvate ATP Pyruvate C C C

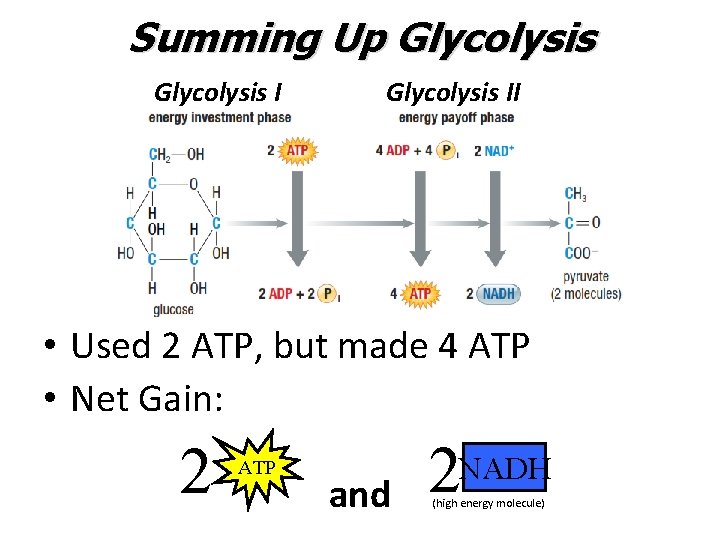
Summing Up Glycolysis II • Used 2 ATP, but made 4 ATP • Net Gain: 2 ATP and 2 NADH (high energy molecule)

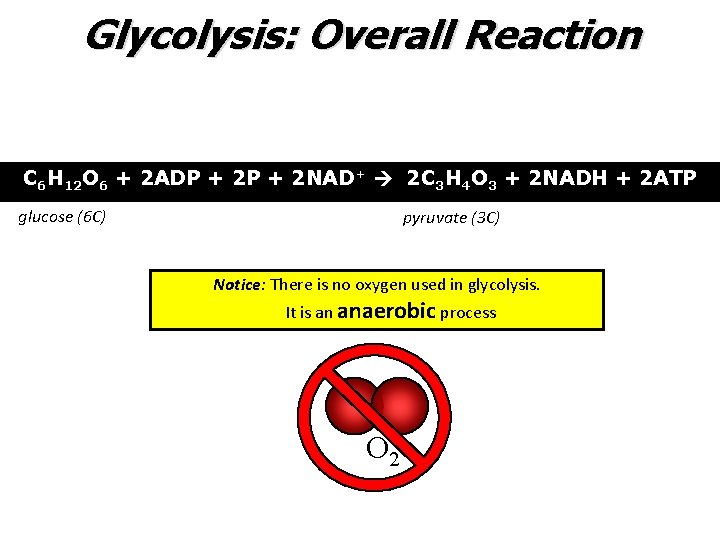
Glycolysis: Overall Reaction C 6 H 12 O 6 + 2 ADP + 2 NAD+ 2 C 3 H 4 O 3 + 2 NADH + 2 ATP glucose (6 C) pyruvate (3 C) Notice: There is no oxygen used in glycolysis. It is an anaerobic process O 2

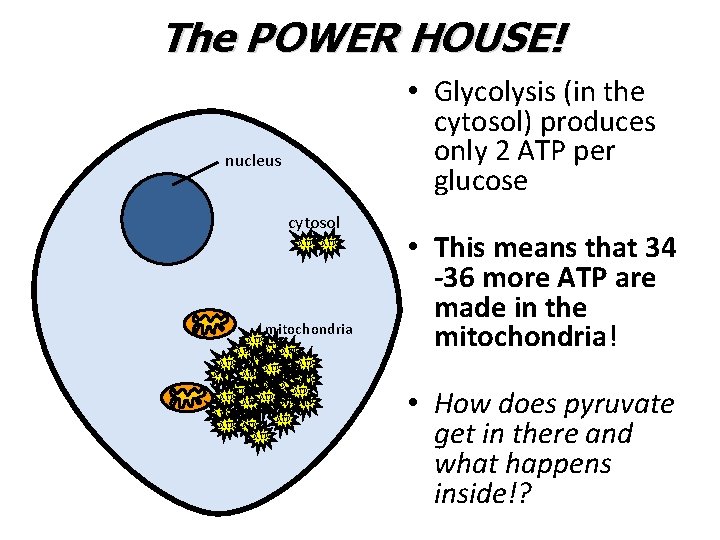
The POWER HOUSE! • Glycolysis (in the cytosol) produces only 2 ATP per glucose nucleus cytosol ATP mitochondria ATP ATP ATP ATP ATP ATP ATP ATP ATP • This means that 34 -36 more ATP are made in the mitochondria! • How does pyruvate get in there and what happens inside!?

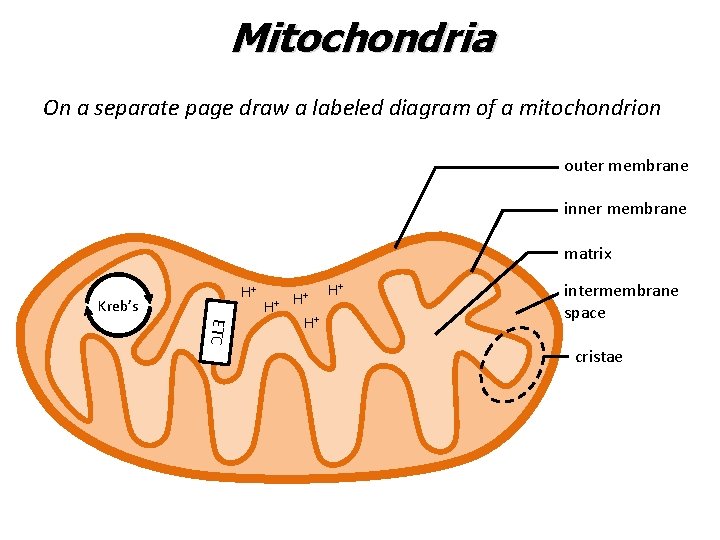
Mitochondria On a separate page draw a labeled diagram of a mitochondrion outer membrane inner membrane matrix H+ Kreb’s H+ H+ ETC H+ H+ intermembrane space cristae

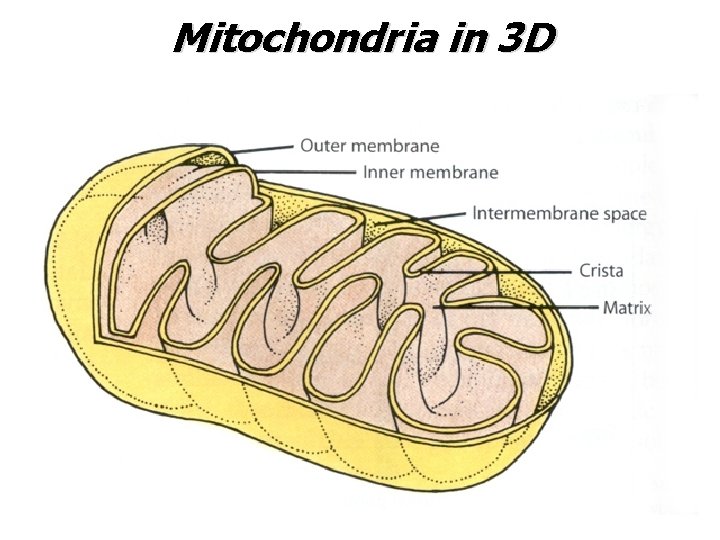
Mitochondria in 3 D

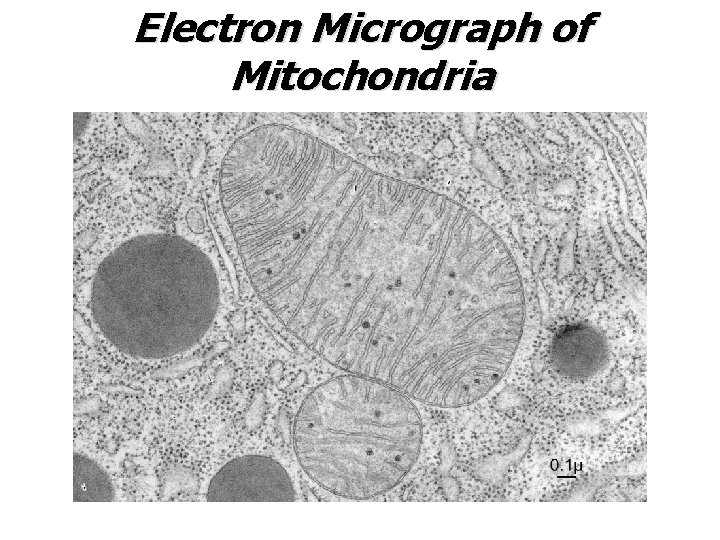
Electron Micrograph of Mitochondria

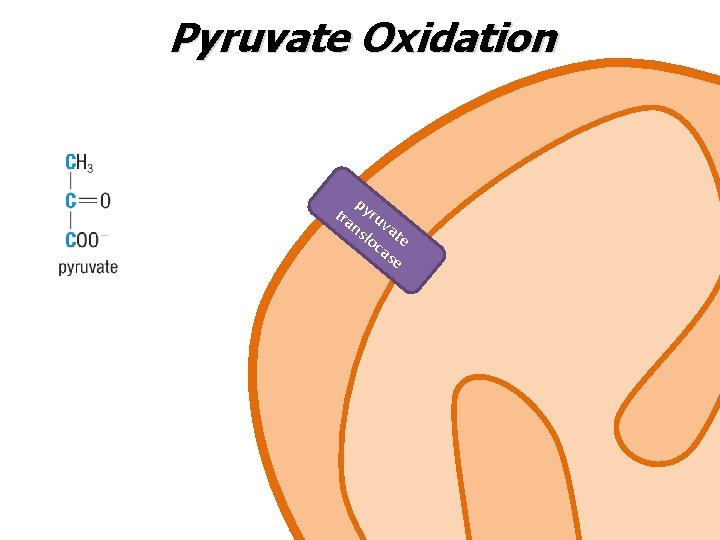
Pyruvate Oxidation p tra yru ns vat loc e as e

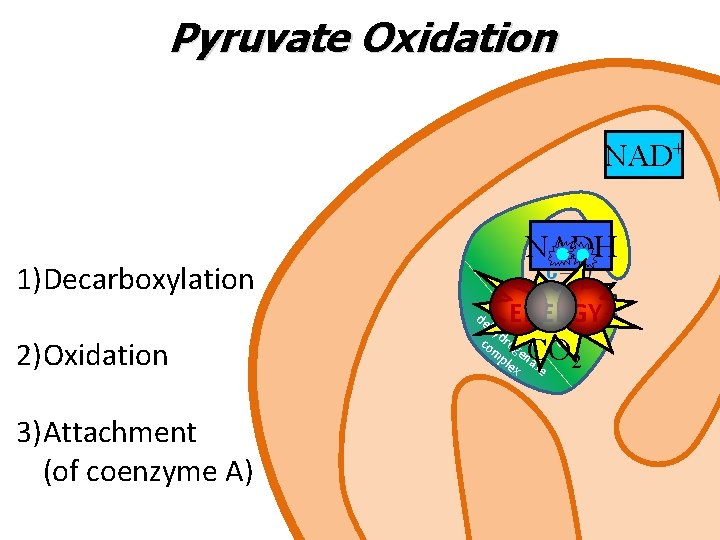
Pyruvate Oxidation NAD+ 1)Decarboxylation NADH m ult py i-en hy ruv zym d co rog ate e m en ple a x se de 2)Oxidation 3)Attachment (of coenzyme A) ENERGY CO 2

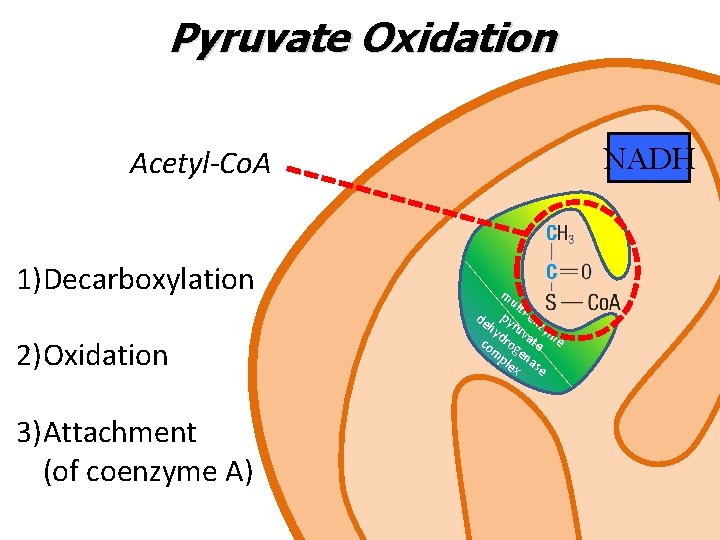
Pyruvate Oxidation NADH Acetyl-Co. A 1)Decarboxylation m ult py i-en hy ruv zym d co rog ate e m en ple a x se de 2)Oxidation 3)Attachment (of coenzyme A)

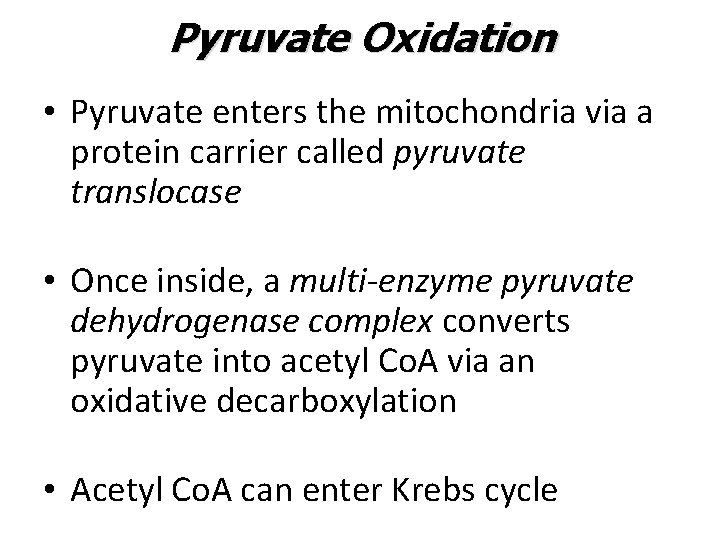
Pyruvate Oxidation • Pyruvate enters the mitochondria via a protein carrier called pyruvate translocase • Once inside, a multi-enzyme pyruvate dehydrogenase complex converts pyruvate into acetyl Co. A via an oxidative decarboxylation • Acetyl Co. A can enter Krebs cycle

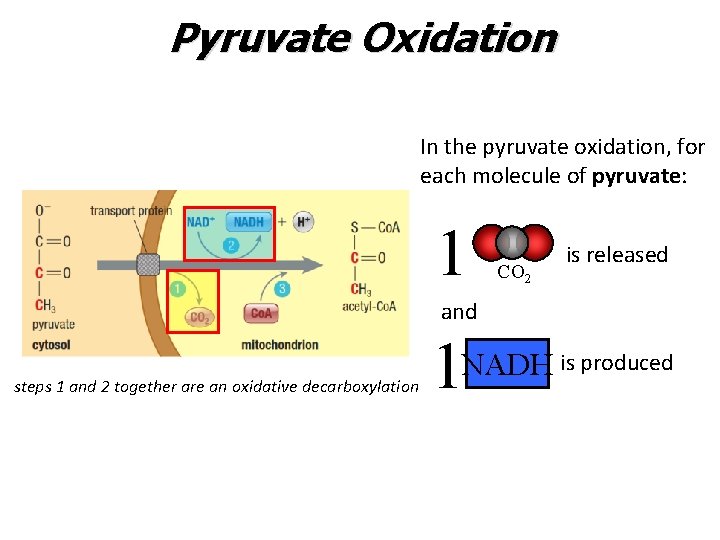
Pyruvate Oxidation In the pyruvate oxidation, for each molecule of pyruvate: 1 CO 2 is released and steps 1 and 2 together are an oxidative decarboxylation 1 NADH is produced

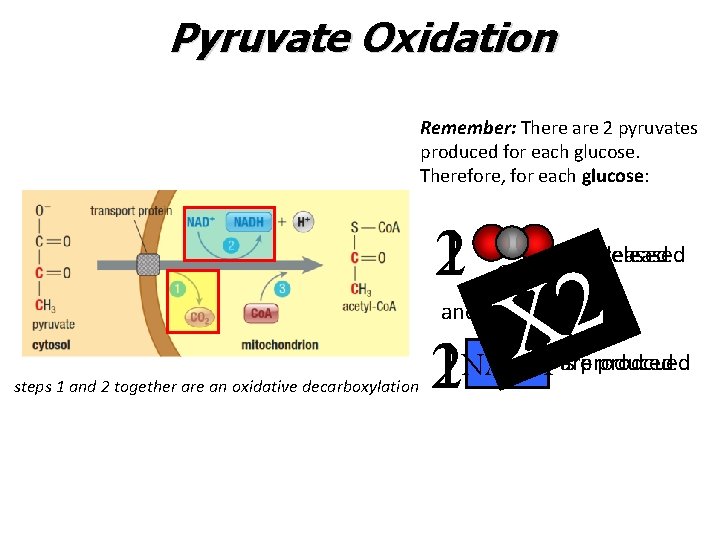
Pyruvate Oxidation Remember: There are 2 pyruvates produced for each glucose. Therefore, for each glucose: 12 and steps 1 and 2 together are an oxidative decarboxylation 12 arereleased is 2 X CO 2 prodcued NADH isareproduced

Krebs / Citric Acid Cycle Pyruvate Oxidation Krebs Cycle

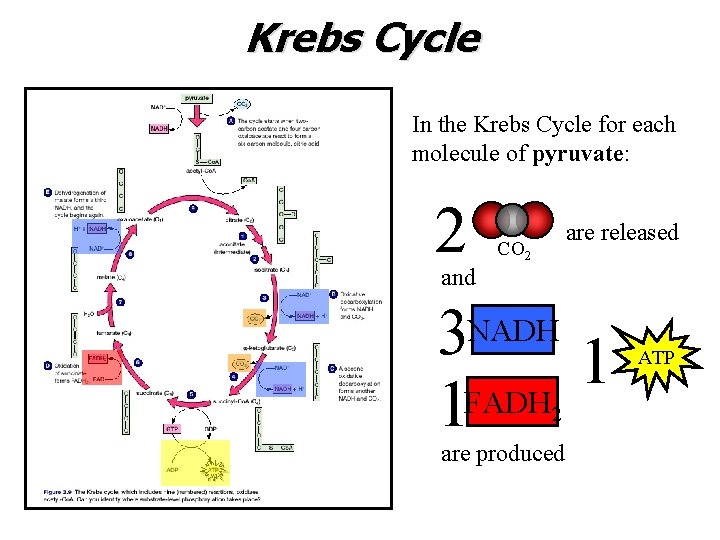
Krebs Cycle In the Krebs Cycle for each molecule of pyruvate: 2 are released CO 2 and 3 NADH 1 1 FADH 2 are produced ATP

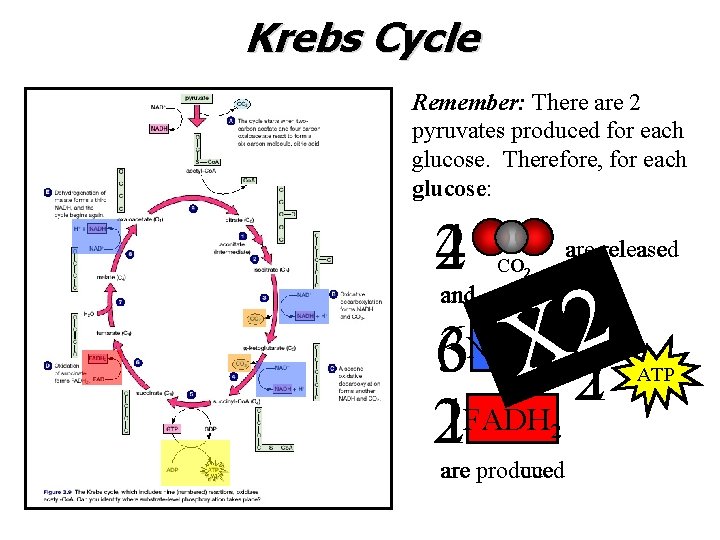
Krebs Cycle Remember: There are 2 pyruvates produced for each glucose. Therefore, for each glucose: 42 and are released CO 2 2 X 63 1 2 21 FADH NADH 2 prodcued are produced ATP

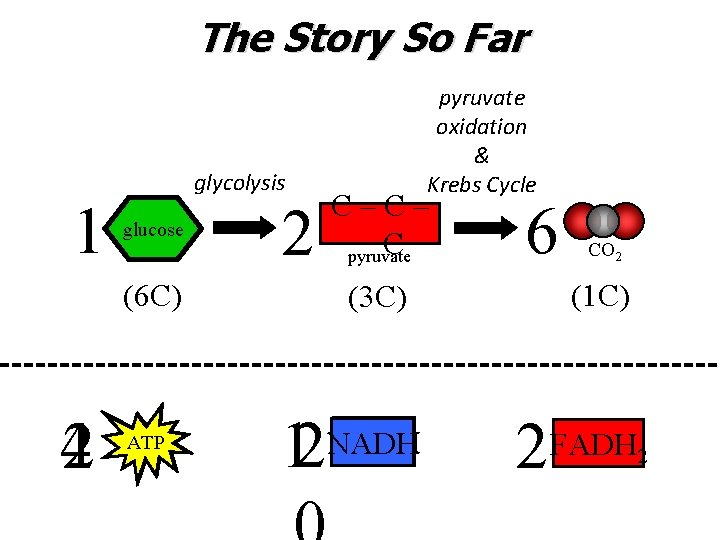
The Story So Far 1 glycolysis glucose (6 C) 42 ATP 2 pyruvate oxidation & Krebs Cycle C–C– C pyruvate (3 C) 12 NADH 6 CO 2 (1 C) 2 FADH 2

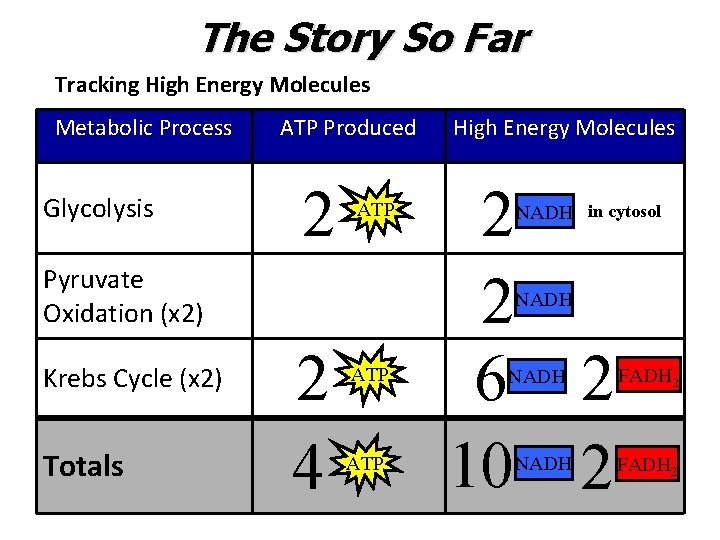
The Story So Far Tracking High Energy Molecules Metabolic Process Glycolysis ATP Produced 2 ATP Pyruvate Oxidation (x 2) Krebs Cycle (x 2) Totals High Energy Molecules 2 2 6 2 10 2 NADH in cytosol NADH 2 4 ATP NADH FADH 2

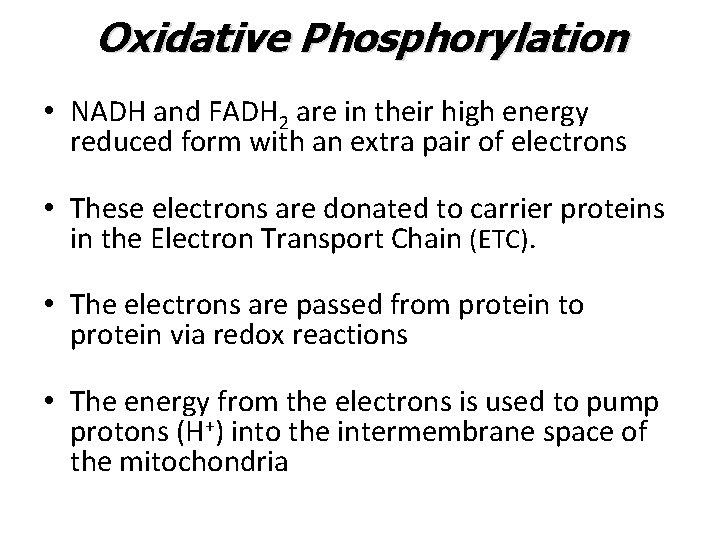
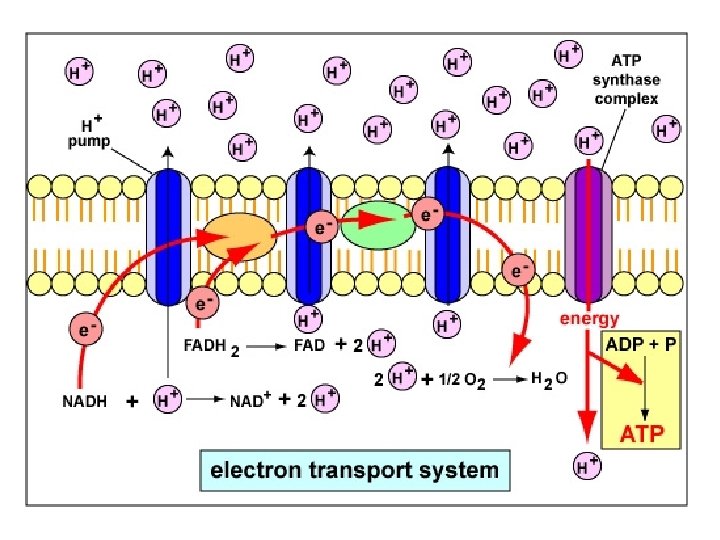
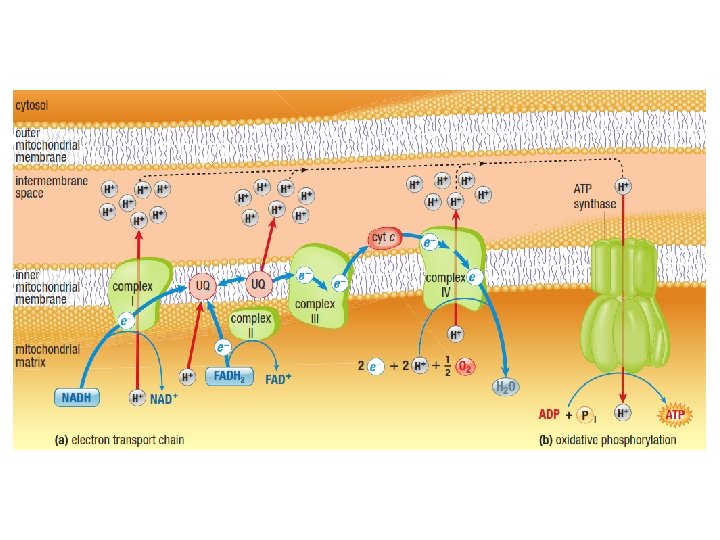
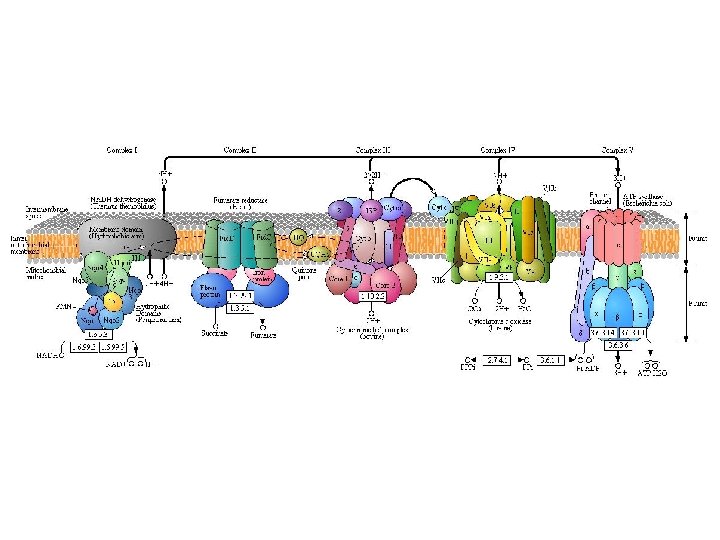
Oxidative Phosphorylation • NADH and FADH 2 are in their high energy reduced form with an extra pair of electrons • These electrons are donated to carrier proteins in the Electron Transport Chain (ETC). • The electrons are passed from protein to protein via redox reactions • The energy from the electrons is used to pump protons (H+) into the intermembrane space of the mitochondria

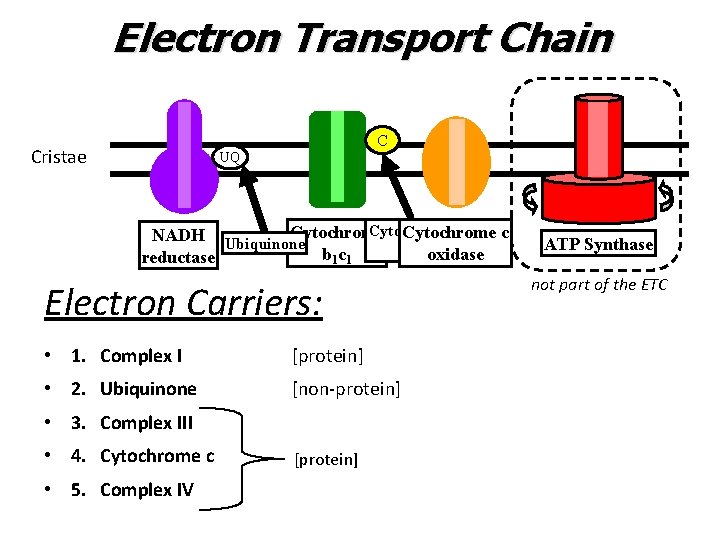
Electron Transport Chain C Cristae UQ Cytochrome c NADH Ubiquinone c oxidase b 1 c 1 reductase Electron Carriers: • 1. Complex I [protein] • 2. Ubiquinone [non-protein] • 3. Complex III • 4. Cytochrome c • 5. Complex IV [protein] ATP Synthase not part of the ETC

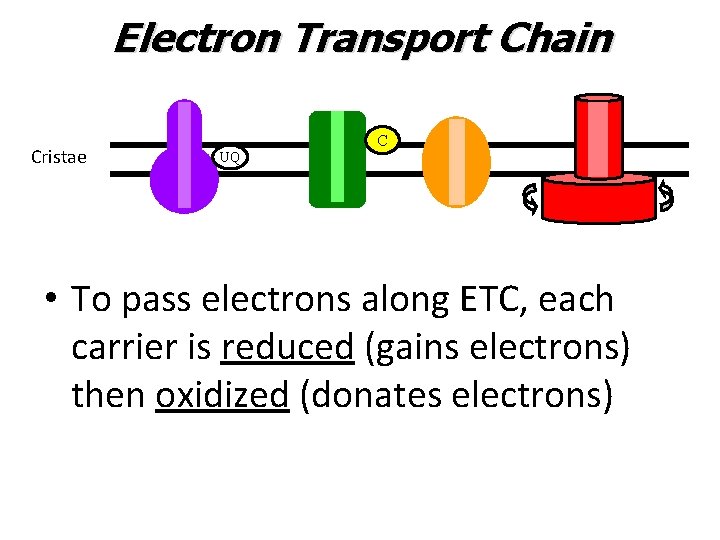
Electron Transport Chain Cristae C UQ • To pass electrons along ETC, each carrier is reduced (gains electrons) then oxidized (donates electrons)

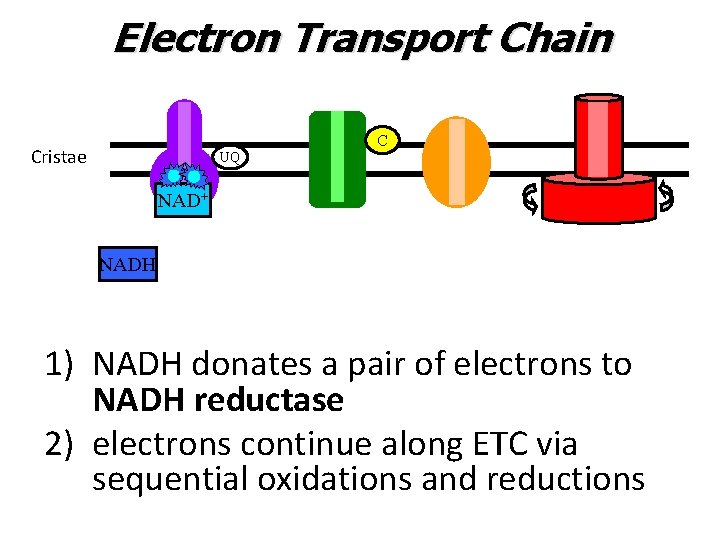
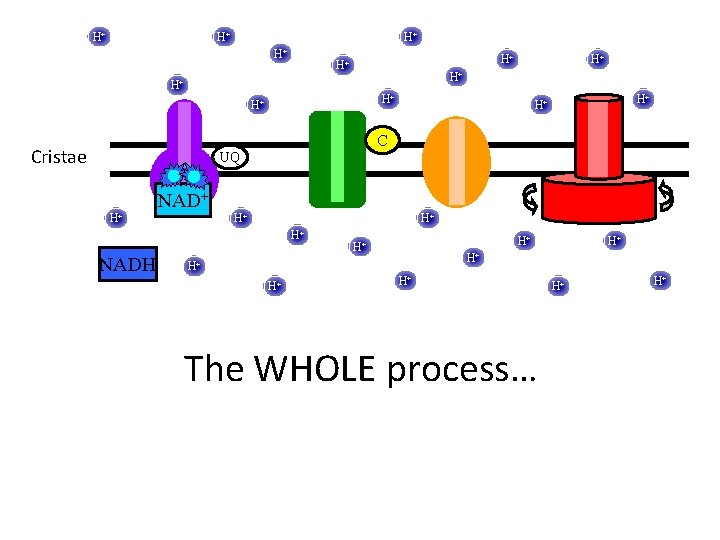
Electron Transport Chain C Cristae UQ NAD+ NADH 1) NADH donates a pair of electrons to NADH reductase 2) electrons continue along ETC via sequential oxidations and reductions

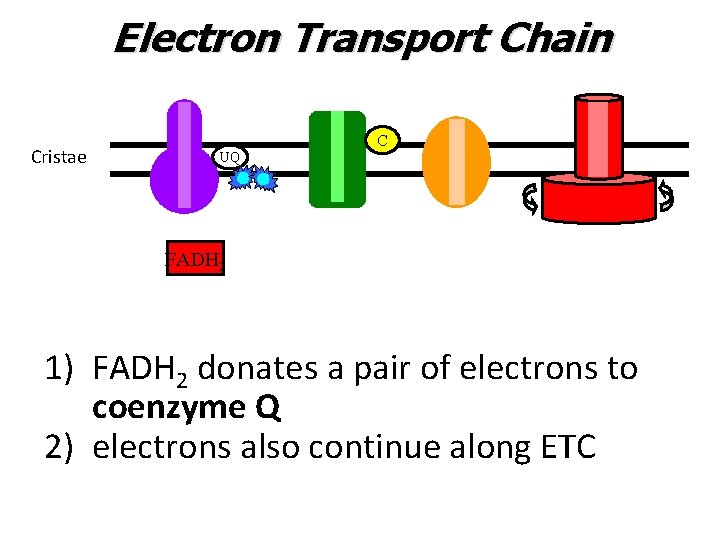
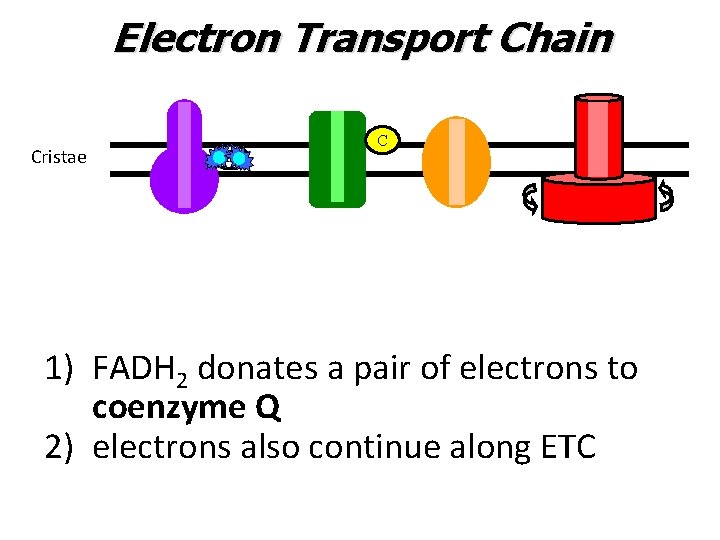
Electron Transport Chain Cristae C UQ FADH 2 1) FADH 2 donates a pair of electrons to coenzyme Q 2) electrons also continue along ETC

Electron Transport Chain Cristae C UQ 1) FADH 2 donates a pair of electrons to coenzyme Q 2) electrons also continue along ETC

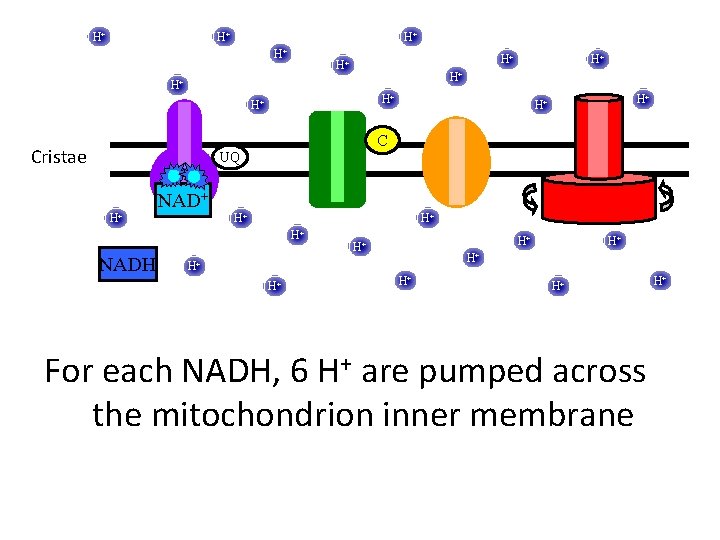
H+ H+ H+ H+ C Cristae UQ H+ NAD+ H+ H+ H+ NADH H+ H+ For each NADH, 6 H+ are pumped across the mitochondrion inner membrane H+

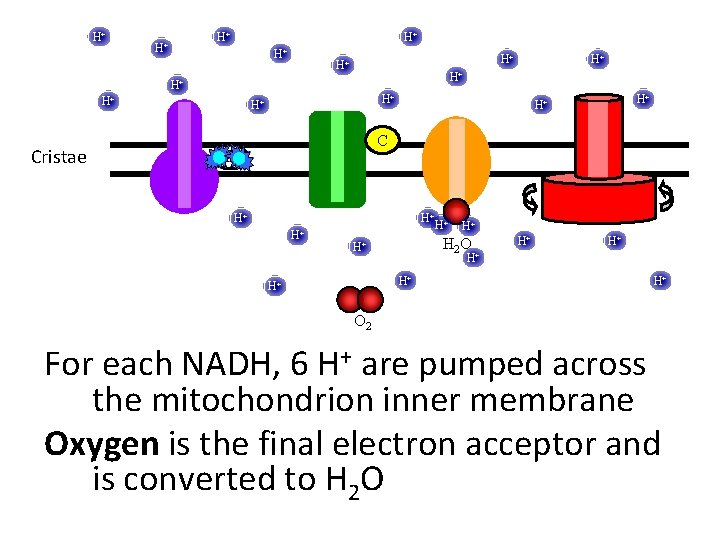
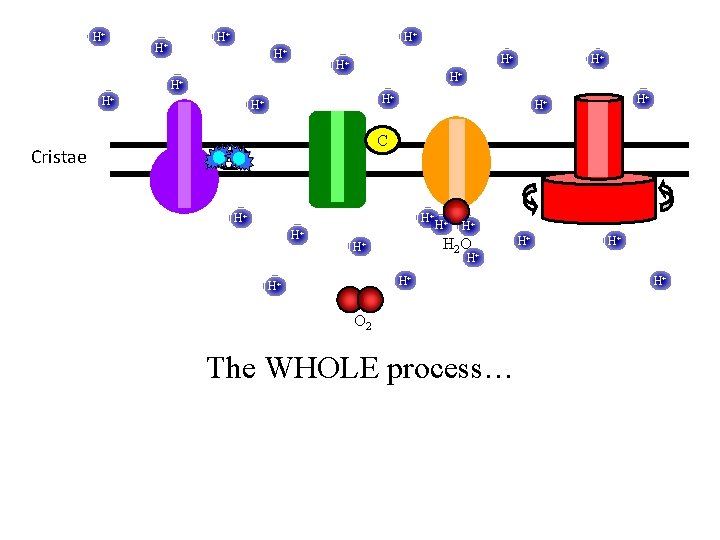
H+ H+ H+ Cristae H+ H+ H+ C UQ H+ H+ H 2 O H+ H+ O 2 For each NADH, 6 H+ are pumped across the mitochondrion inner membrane Oxygen is the final electron acceptor and is converted to H 2 O

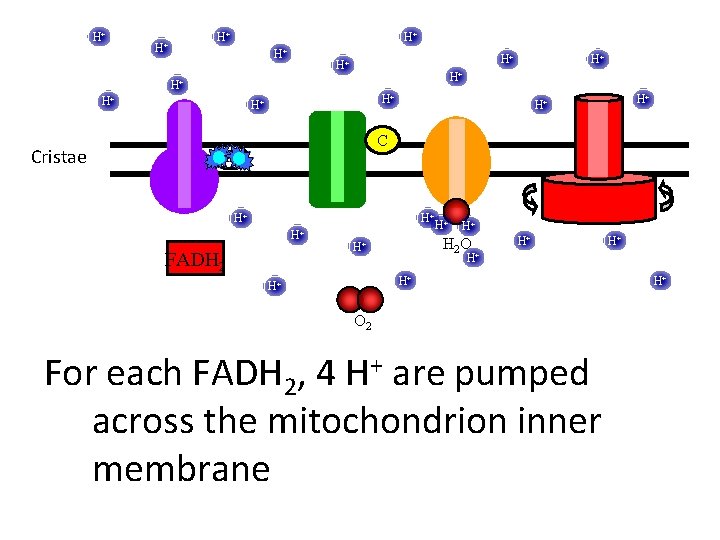
H+ H+ H+ Cristae H+ H+ H+ C UQ H+ H+ H+ FADH 2 H+ H+ H 2 O H+ H+ H+ O 2 For each FADH 2, 4 H+ are pumped across the mitochondrion inner membrane H+

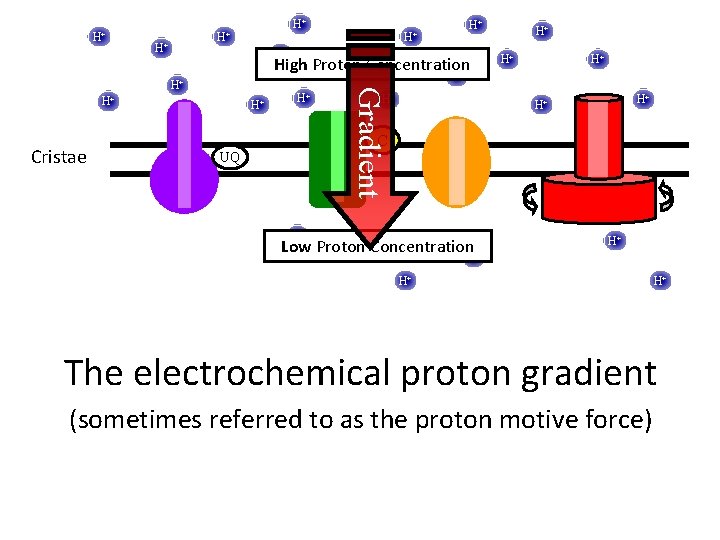
H+ H+ H+ Concentration High Proton + H+ H+ H+ Gradient H+ Cristae H+ H+ H+ C UQ H+ H+ Concentration Low Proton + H+ H+ The electrochemical proton gradient (sometimes referred to as the proton motive force)

• ATP synthase works a bit like a water mill

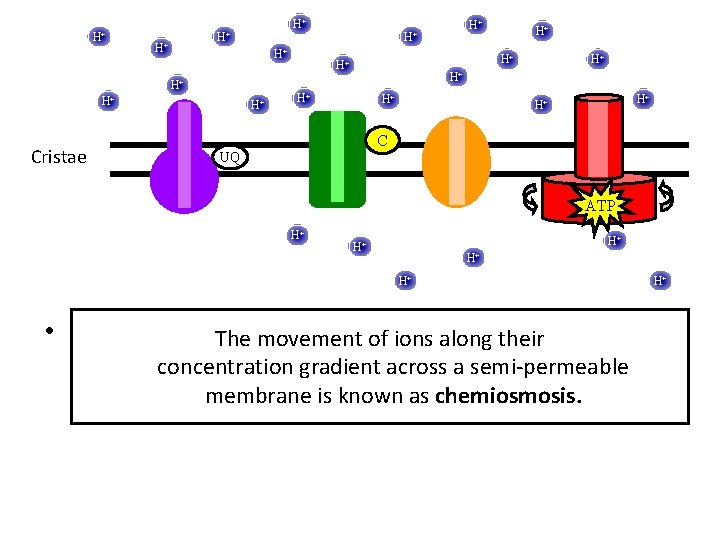
H+ H+ Cristae H+ H+ H+ C UQ ATP H+ H+ H+ • H+ Using the energy stored of in ions the along proton gradient, ATP The movement their is generated usinggradient oxidative phosphorylation: concentration across a semi-permeable is known chemiosmosis. formationmembrane of ATP coupled toasoxygen consumption

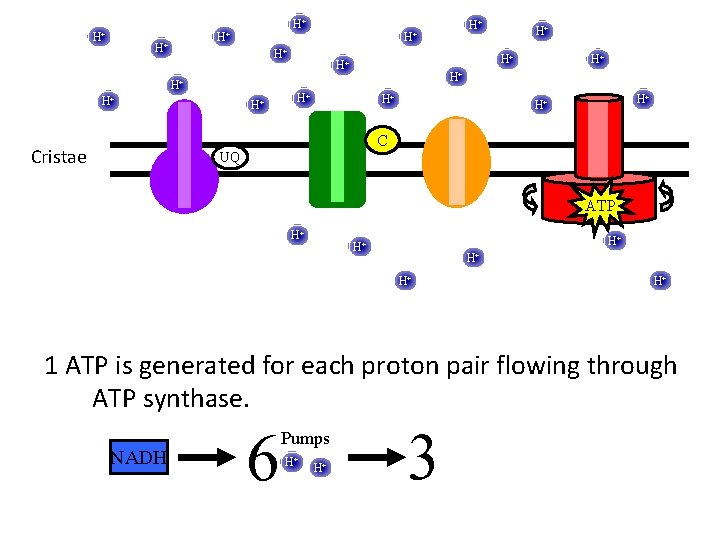
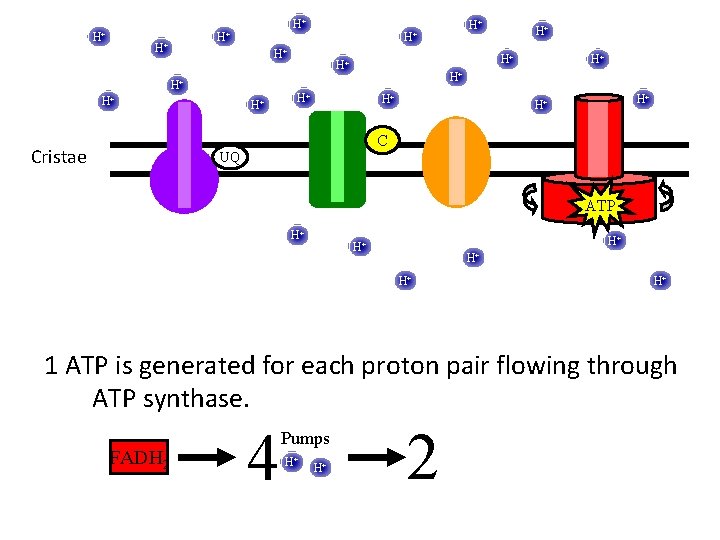
H+ H+ H+ H+ H+ C Cristae UQ ATP H+ H+ H+ 1 ATP is generated for each proton pair flowing through ATP synthase. NADH 6 Pumps H+ H+ 3

H+ H+ H+ H+ H+ C Cristae UQ ATP H+ H+ H+ 1 ATP is generated for each proton pair flowing through ATP synthase. FADH 2 4 Pumps H+ H+ 2

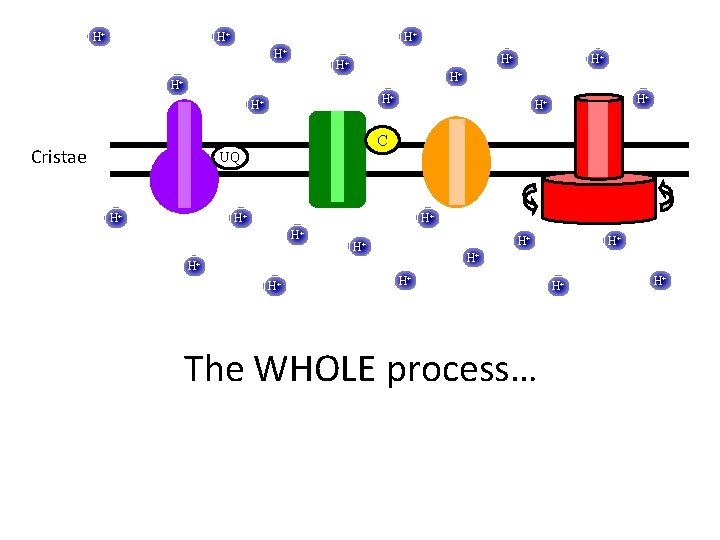
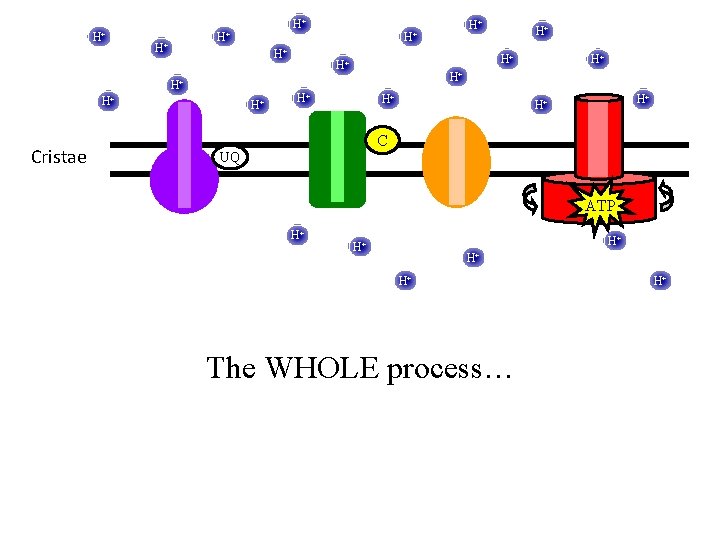
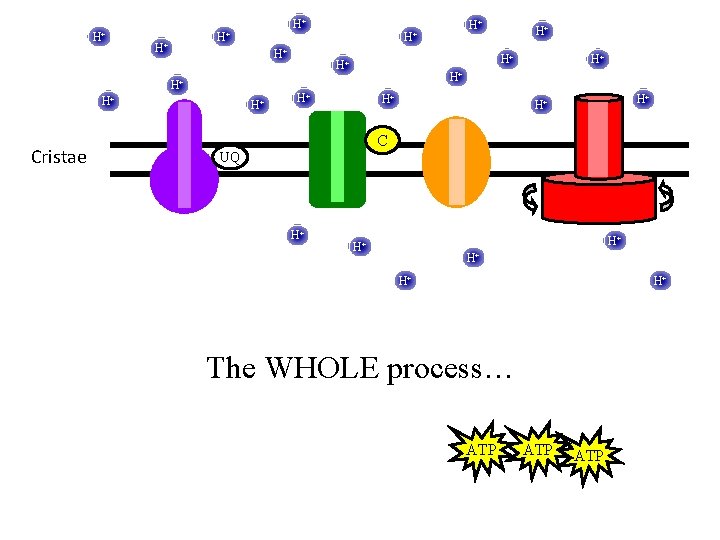
H+ H+ H+ H+ C Cristae UQ H+ H+ H+ The WHOLE process… H+ H+

H+ H+ H+ H+ C Cristae UQ H+ NAD+ H+ H+ H+ NADH H+ H+ The WHOLE process… H+ H+

H+ H+ H+ Cristae H+ H+ H+ C UQ H+ H+ H+ H 2 O H+ H+ H+ O 2 The WHOLE process… H+

H+ H+ Cristae H+ H+ H+ C UQ ATP H+ H+ H+ The WHOLE process… H+

H+ H+ Cristae H+ H+ H+ C UQ H+ H+ H+ The WHOLE process… ATP ATP




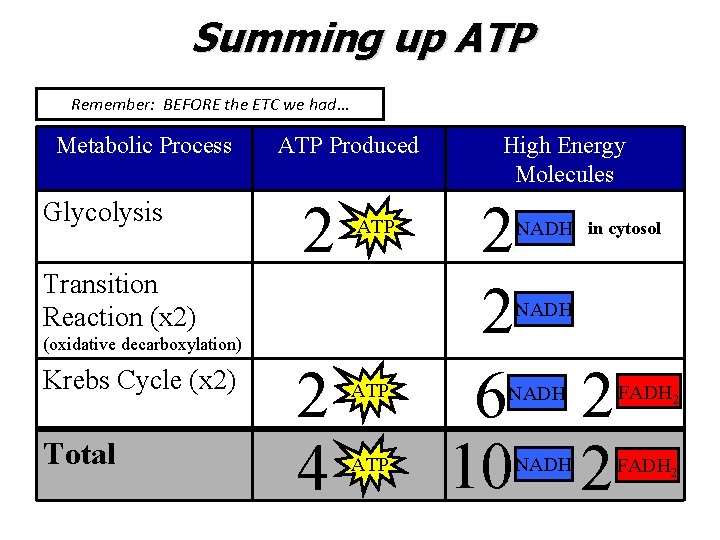
Summing up ATP Remember: BEFORE the ETC we had… Metabolic Process Glycolysis ATP Produced 2 ATP Transition Reaction (x 2) Total 2 2 6 2 10 2 NADH in cytosol NADH (oxidative decarboxylation) Krebs Cycle (x 2) High Energy Molecules 2 4 ATP NADH FADH 2

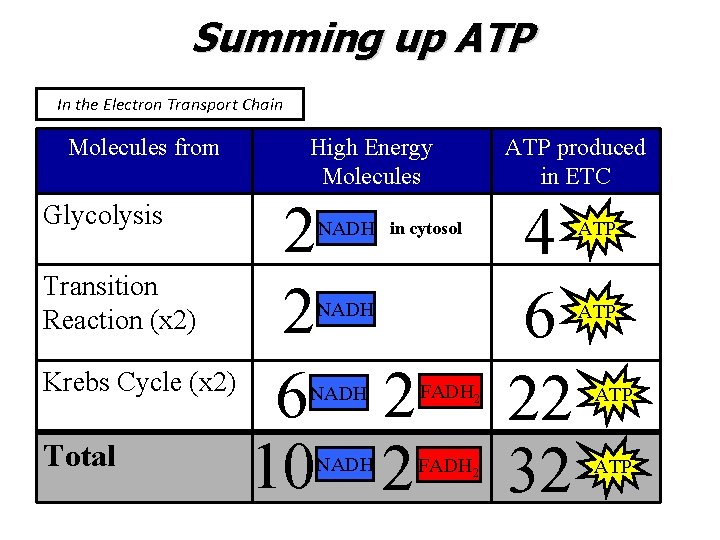
Summing up ATP In the Electron Transport Chain Molecules from High Energy Molecules 2 Transition Reaction (x 2) 2 Krebs Cycle (x 2) 6 2 Total 10 2 Glycolysis NADH in cytosol NADH FADH 2 ATP produced in ETC 4 6 22 32 ATP ATP

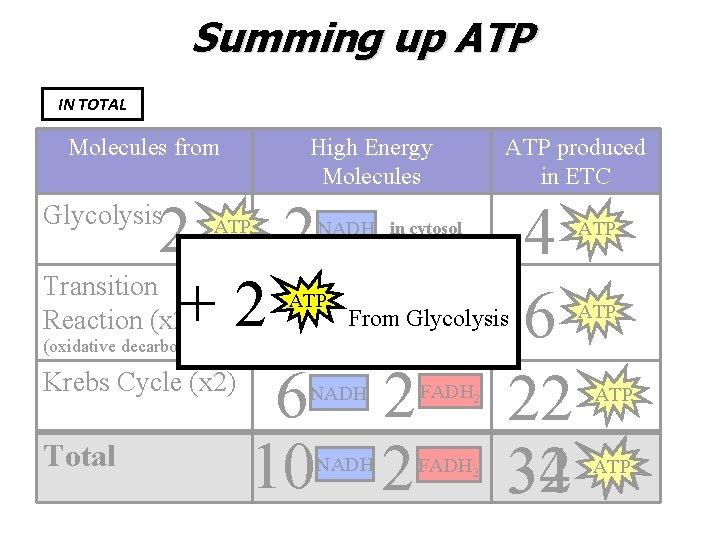
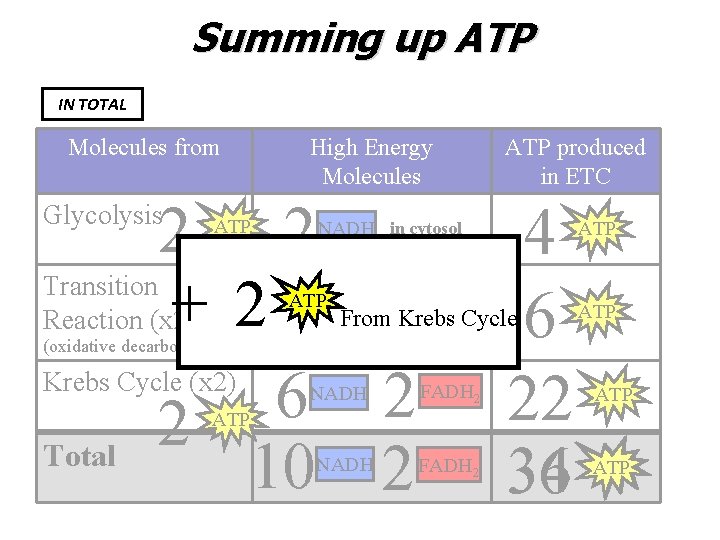
Summing up ATP IN TOTAL Molecules from High Energy Molecules 2 2 Transition +22 Reaction (x 2) Krebs Cycle (x 2) 6 2 Total 10 2 Glycolysis ATP NADH in cytosol ATPNADH From NADH 4 6 22 32 34 Glycolysis (oxidative decarboxylation) NADH ATP produced in ETC FADH 2 ATP ATP

Summing up ATP IN TOTAL Molecules from High Energy Molecules 2 2 Transition + 22 Reaction (x 2) Krebs Cycle (x 2) 6 2 2 Total 10 2 Glycolysis ATP produced in ETC NADH in cytosol ATPNADH From Krebs Cycle (oxidative decarboxylation) NADH FADH 2 ATP NADH 4 6 22 34 36 FADH 2 ATP ATP

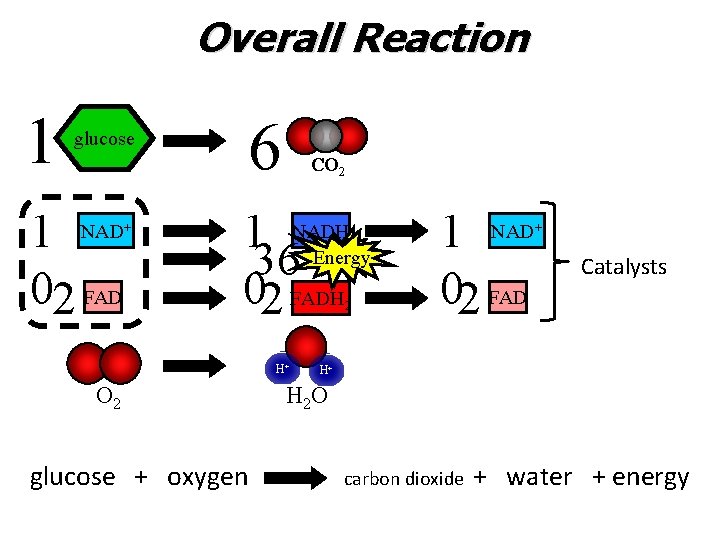
Overall Reaction 1 6 glucose 1 02 FAD NAD+ CO 2 1 36 02 FADH NADH ATP Energy H+ O 2 glucose + oxygen 2 1 02 FAD NAD+ Catalysts H+ H 2 O carbon dioxide + water + energy

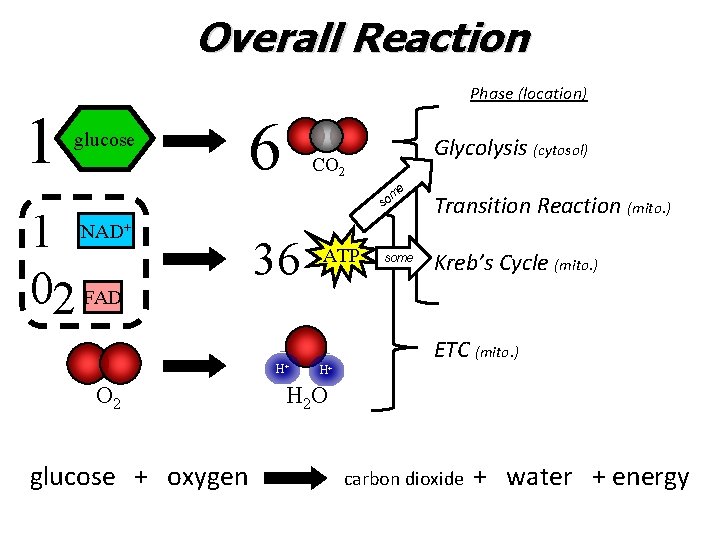
Overall Reaction Phase (location) 1 glucose 6 Glycolysis (cytosol) CO 2 e 1 02 FAD NAD+ 36 H+ O 2 glucose + oxygen ATP m so Transition Reaction (mito. ) some Kreb’s Cycle (mito. ) ETC (mito. ) H+ H 2 O carbon dioxide + water + energy

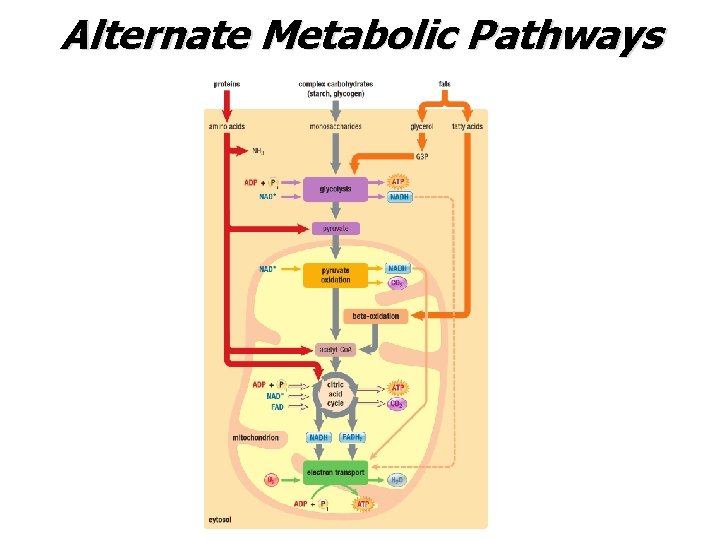
Alternate Metabolic Pathways

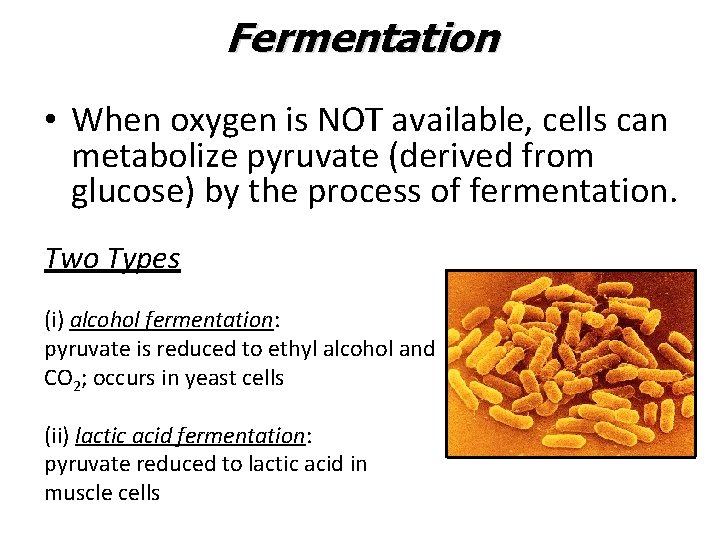
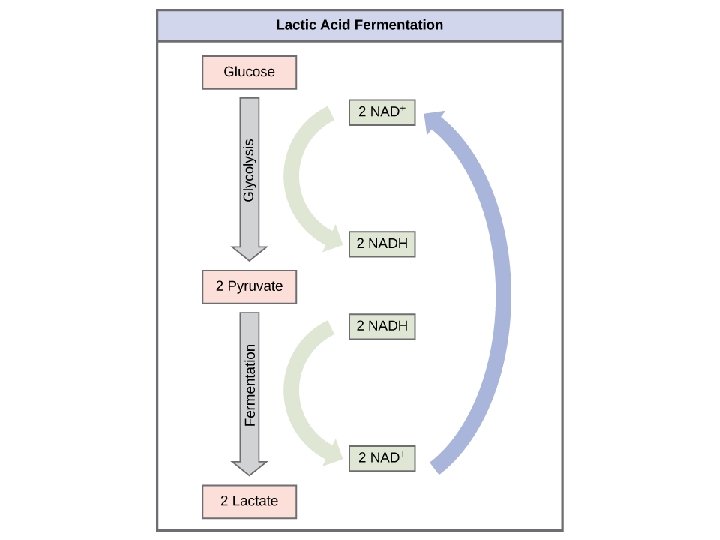
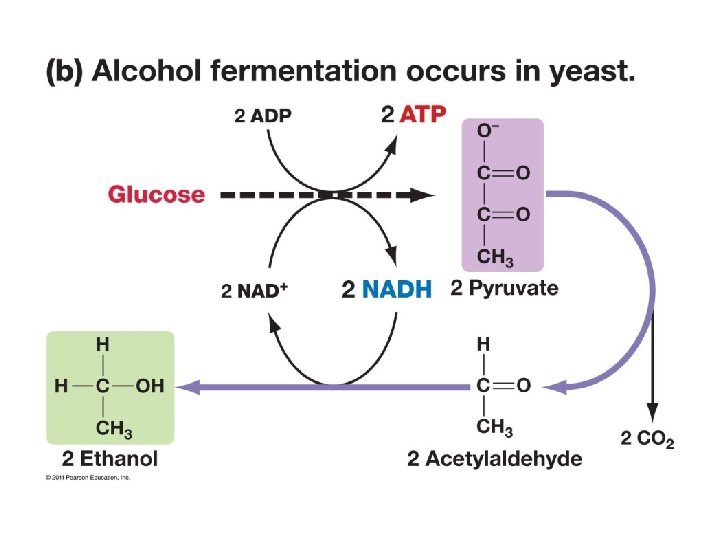
Fermentation • When oxygen is NOT available, cells can metabolize pyruvate (derived from glucose) by the process of fermentation. Two Types (i) alcohol fermentation: pyruvate is reduced to ethyl alcohol and CO 2; occurs in yeast cells (ii) lactic acid fermentation: pyruvate reduced to lactic acid in muscle cells

