CELLULAR RESPIRATION FERMENTATION CHAPTER 7 BIOLOGY IN FOCUS
CELLULAR RESPIRATION & FERMENTATION CHAPTER 7 BIOLOGY IN FOCUS AP BIO 2014
Living systems require energy -- Life is work • Complex organic molecules have potential energy stored in their covalent bonds (think “energized” electrons) • This form of potential energy is called chemical energy • We can use chemical energy to perform cellular work inside our cells • Our cells generate and depend upon one final usable energy source ATP
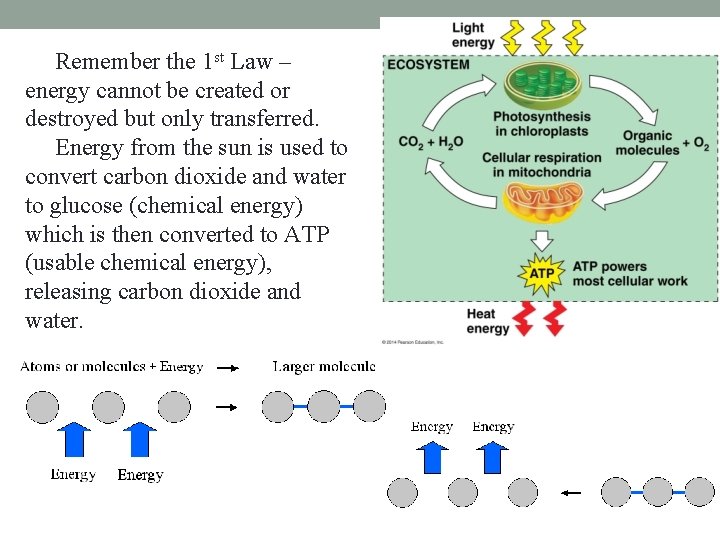
Remember the 1 st Law – energy cannot be created or destroyed but only transferred. Energy from the sun is used to convert carbon dioxide and water to glucose (chemical energy) which is then converted to ATP (usable chemical energy), releasing carbon dioxide and water.
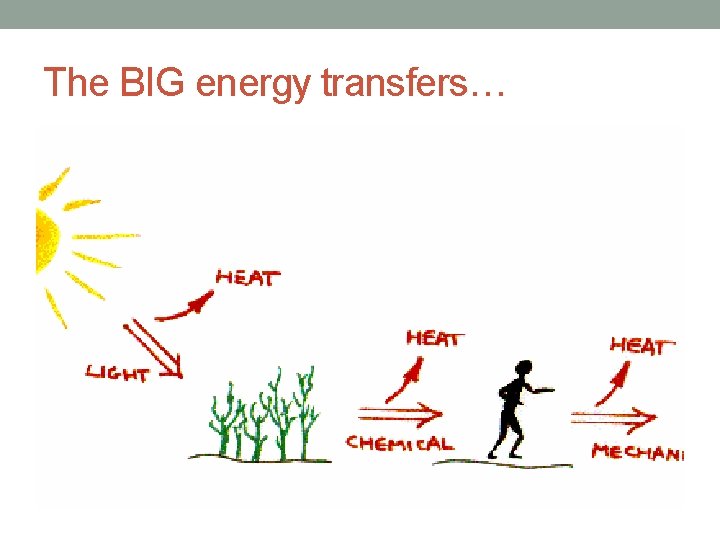
The BIG energy transfers…
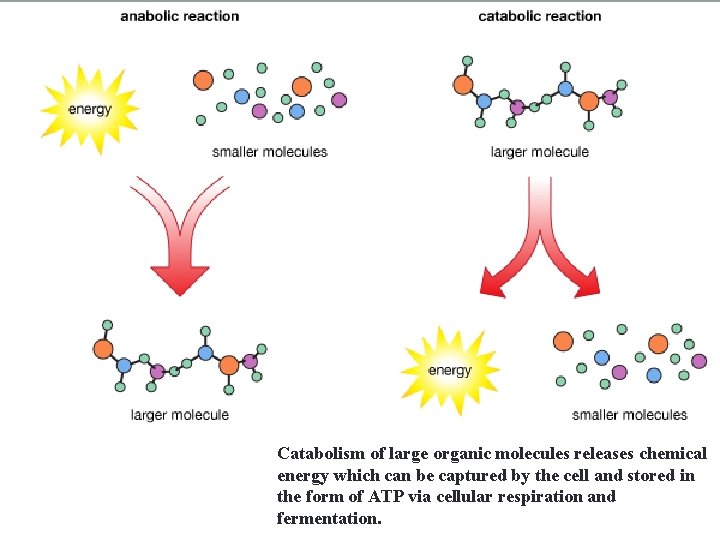
Catabolism of large organic molecules releases chemical energy which can be captured by the cell and stored in the form of ATP via cellular respiration and fermentation.
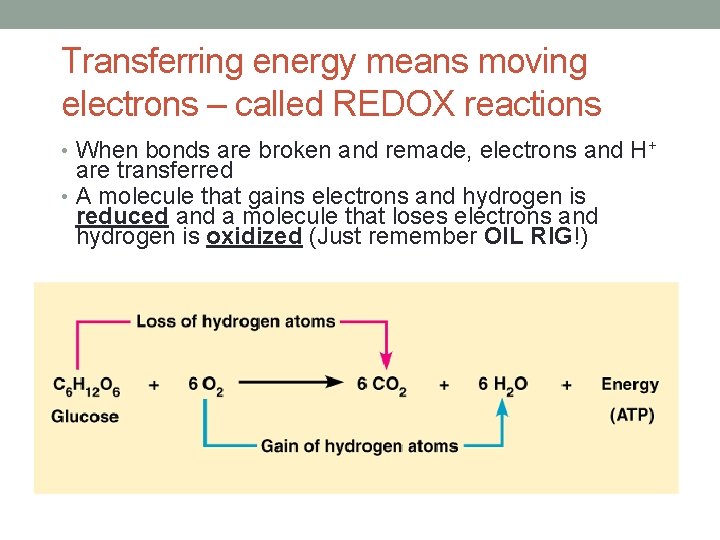
Transferring energy means moving electrons – called REDOX reactions • When bonds are broken and remade, electrons and H+ are transferred • A molecule that gains electrons and hydrogen is reduced and a molecule that loses electrons and hydrogen is oxidized (Just remember OIL RIG!)
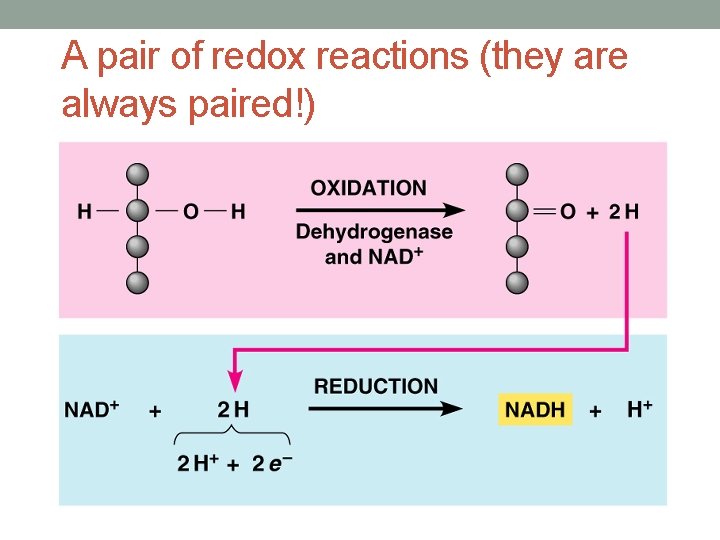
A pair of redox reactions (they are always paired!)

More redox vocab… • The molecule that gives up electrons is called the reducing agent • The molecule that takes the electrons is called an oxidizing agent. Because oxygen is so electronegative, it is one of the most powerful oxidizing agents.
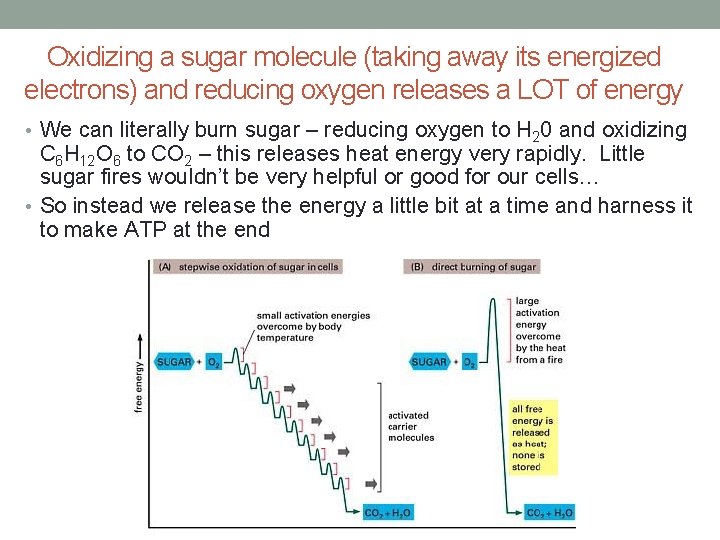
Oxidizing a sugar molecule (taking away its energized electrons) and reducing oxygen releases a LOT of energy • We can literally burn sugar – reducing oxygen to H 20 and oxidizing C 6 H 12 O 6 to CO 2 – this releases heat energy very rapidly. Little sugar fires wouldn’t be very helpful or good for our cells… • So instead we release the energy a little bit at a time and harness it to make ATP at the end
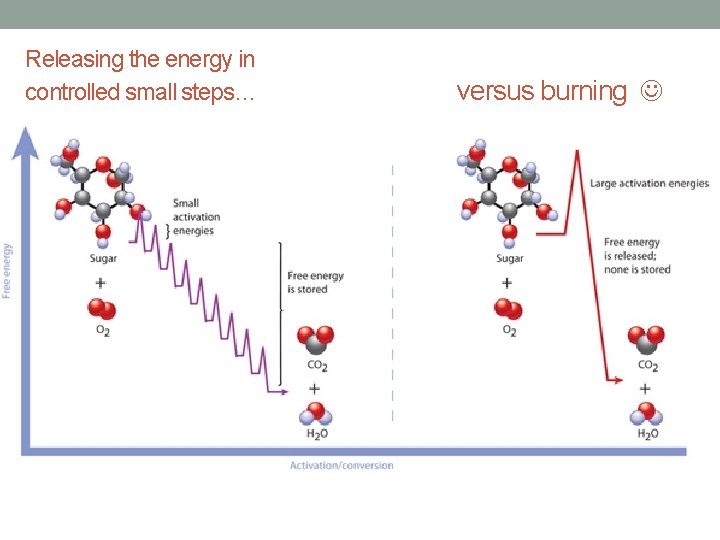
Releasing the energy in controlled small steps… versus burning
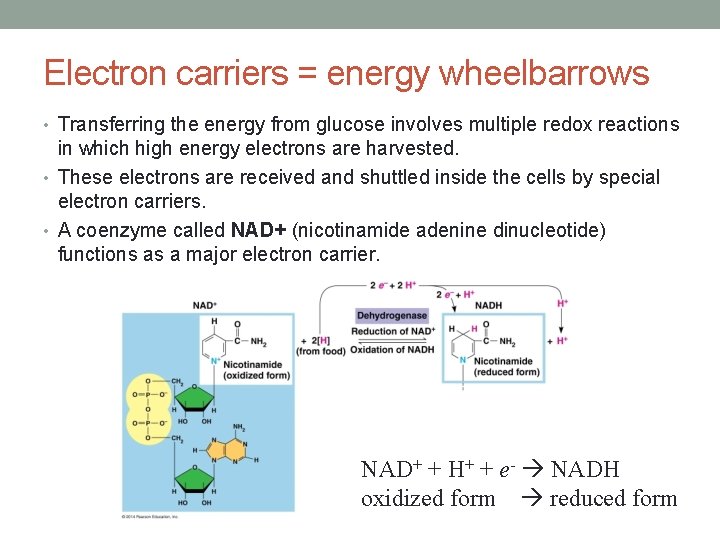
Electron carriers = energy wheelbarrows • Transferring the energy from glucose involves multiple redox reactions in which high energy electrons are harvested. • These electrons are received and shuttled inside the cells by special electron carriers. • A coenzyme called NAD+ (nicotinamide adenine dinucleotide) functions as a major electron carrier. NAD+ + H+ + e- NADH oxidized form reduced form

Let’s review… • Objective: Objective To harvest the energy stored in food and transfer to a molecule that we can use – ATP • Where is the energy stored? In bonds, in the form of high energy electrons. So we need to get these high energy electrons from food to ATP • When electrons are transferred, it’s called a redox reaction. The molecule that loses the electrons is oxidized and the molecule that gains the electrons is reduced. • The big electron carrier in cellular respiration is NAD+/NADH but it gets some help from FAD/FADH 2
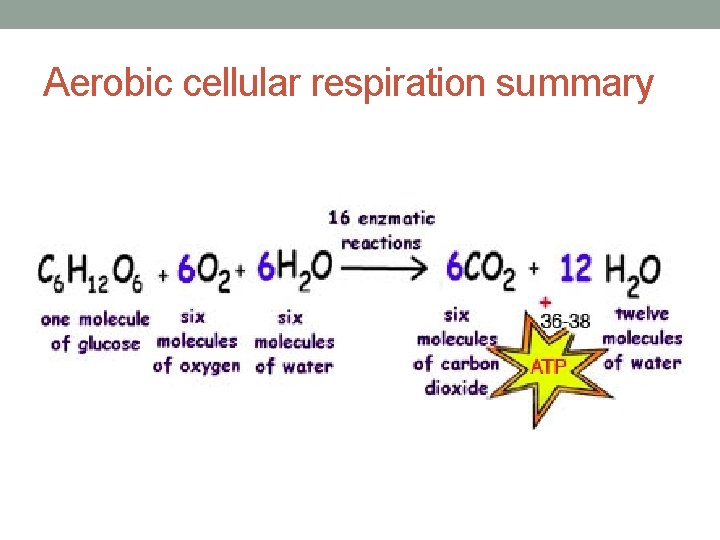
Aerobic cellular respiration summary
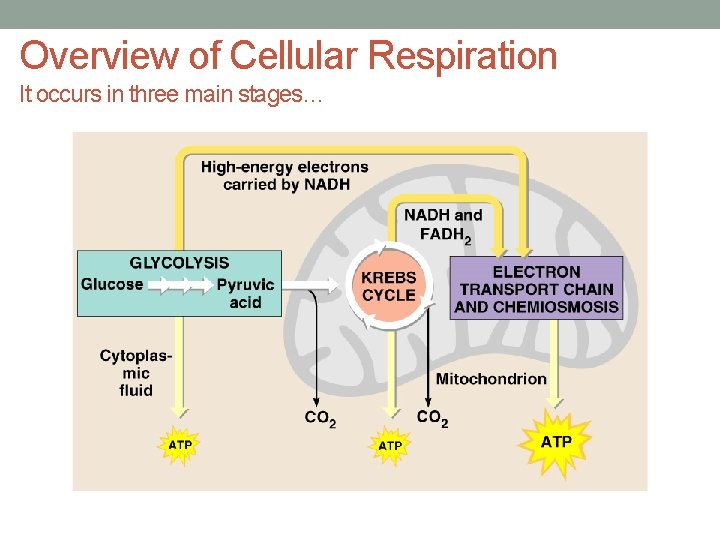
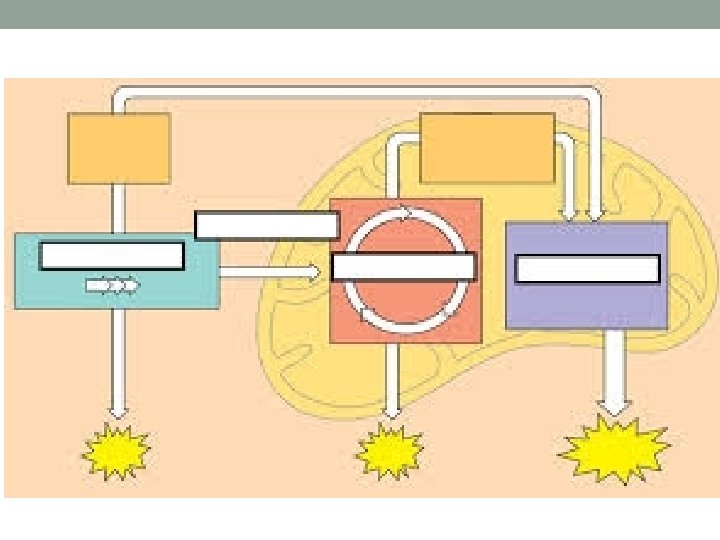
Overview of Cellular Respiration It occurs in three main stages…
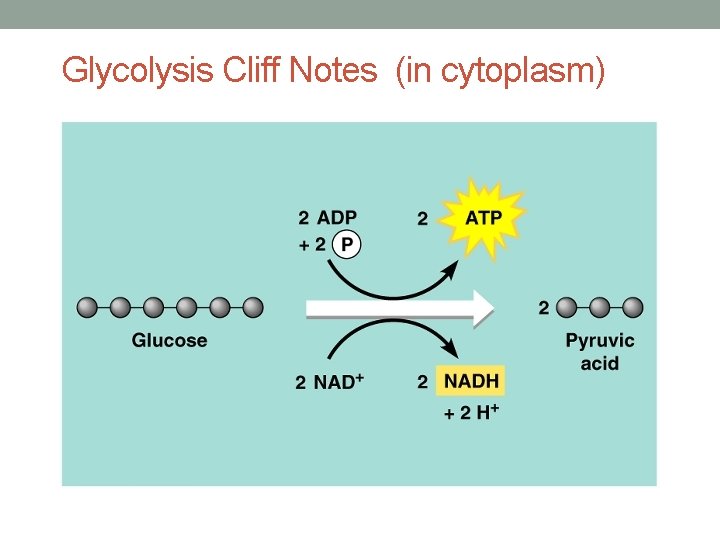
Glycolysis Cliff Notes (in cytoplasm)
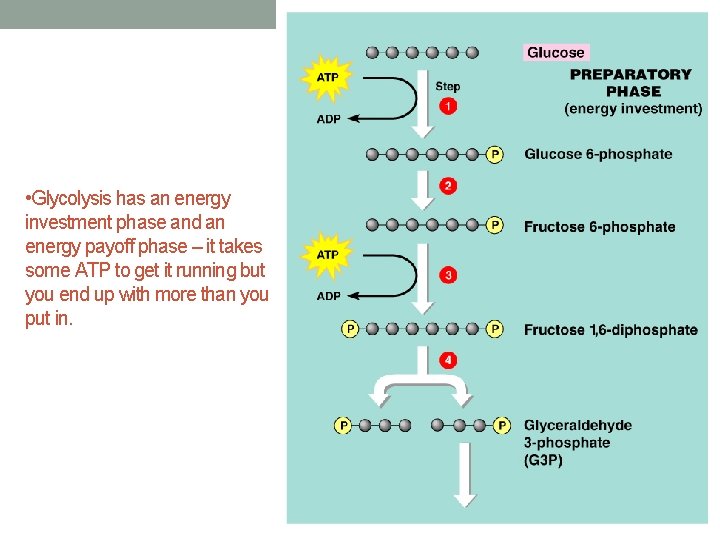
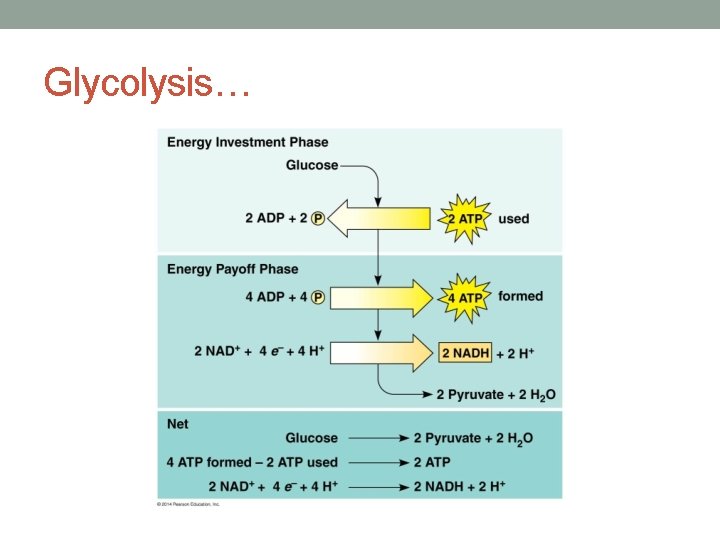
• Glycolysis has an energy investment phase and an energy payoff phase – it takes some ATP to get it running but you end up with more than you put in.
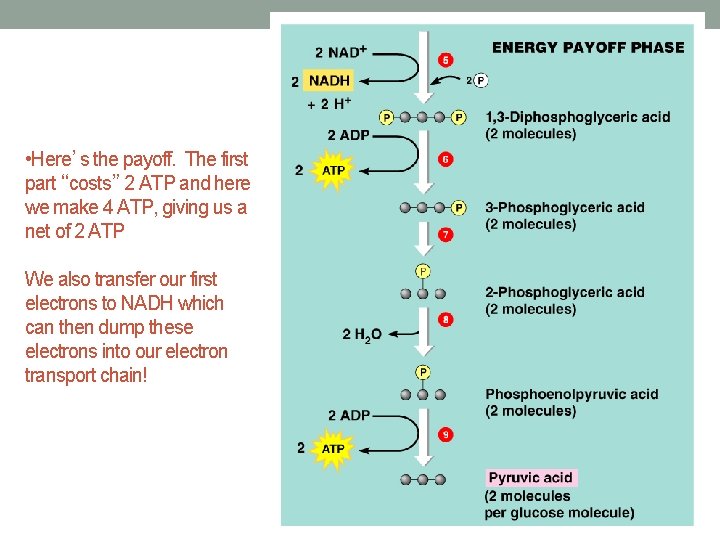
• Here’s the payoff. The first part “costs” 2 ATP and here we make 4 ATP, giving us a net of 2 ATP We also transfer our first electrons to NADH which can then dump these electrons into our electron transport chain!
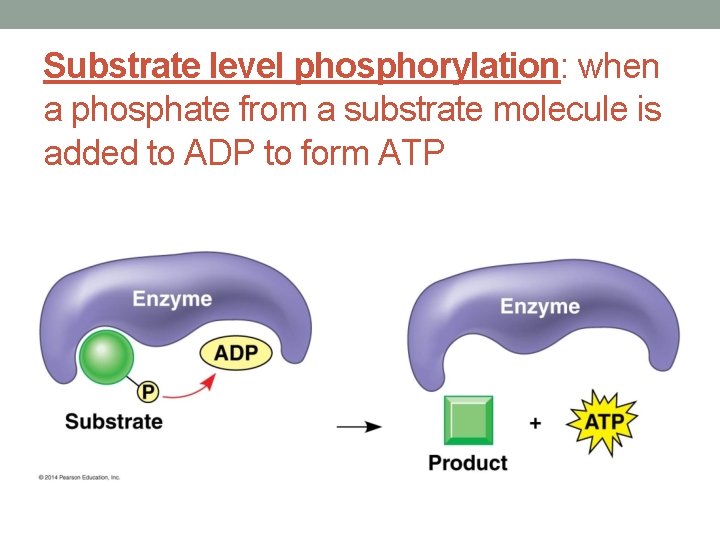
Substrate level phosphorylation: when a phosphate from a substrate molecule is added to ADP to form ATP
Glycolysis…

Glycolysis, in a nut shell… • Converts 6 -carbon glucose to two 3 -carbon pyruvic acids (aka pyruvate) • Produces 2 ATP and 2 NADH • Occurs in the cytoplasm • Does not require oxygen as a reactant! Anaerobic • Prokaryotic cells (bacteria) and eukaryotic cells all perform glycolysis (everybody’s doin’ it…. )
Glycolysis, the movie
Glycolysis videos • https: //www. youtube. com/watch? v=8 Kn 6 BVGq. Kd 8 • Ha! Glycolysis rap: https: //www. youtube. com/watch? v=Ef. Glznwfu 9 U
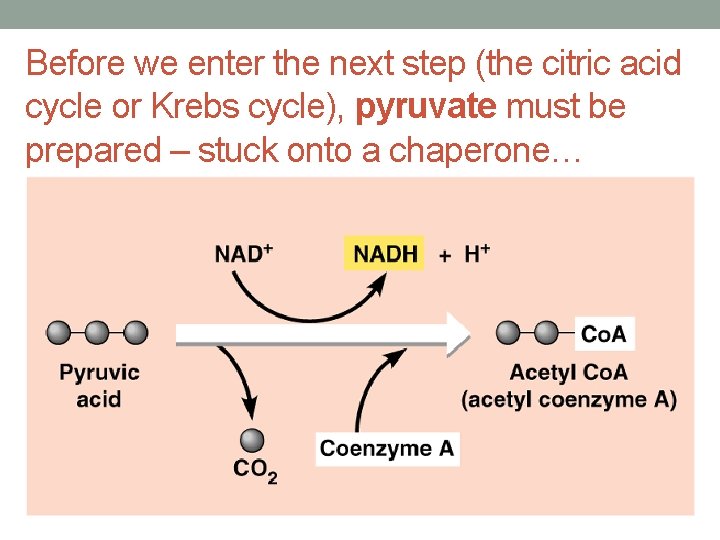
Before we enter the next step (the citric acid cycle or Krebs cycle), pyruvate must be prepared – stuck onto a chaperone…
Step 2: The Citric Acid/Krebs cycle • Occurs in the matrix of the mitochondria (see next slide) • Involves a bohemoth enzyme complex • The point – to finish completely oxidizing what’s left of glucose by transferring the electrons and H+ to NAD+ and FAD • Products per one acetyl. Co. A: 3 NADH, 1 ATP, and 1 FADH 2 and CO 2 as waste. • Remember – there are 2 acetyl-Co. A’s per glucose!
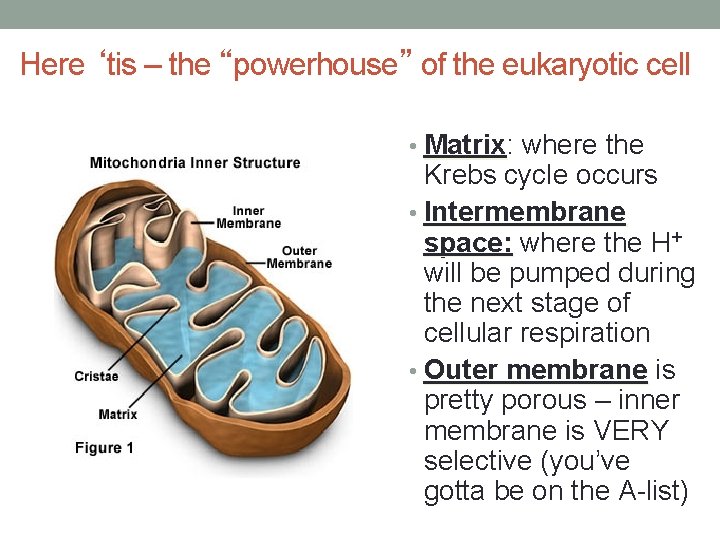
Here ‘tis – the “powerhouse” of the eukaryotic cell • Matrix: Matrix where the Krebs cycle occurs • Intermembrane space: where the H+ will be pumped during the next stage of cellular respiration • Outer membrane is pretty porous – inner membrane is VERY selective (you’ve gotta be on the A-list)
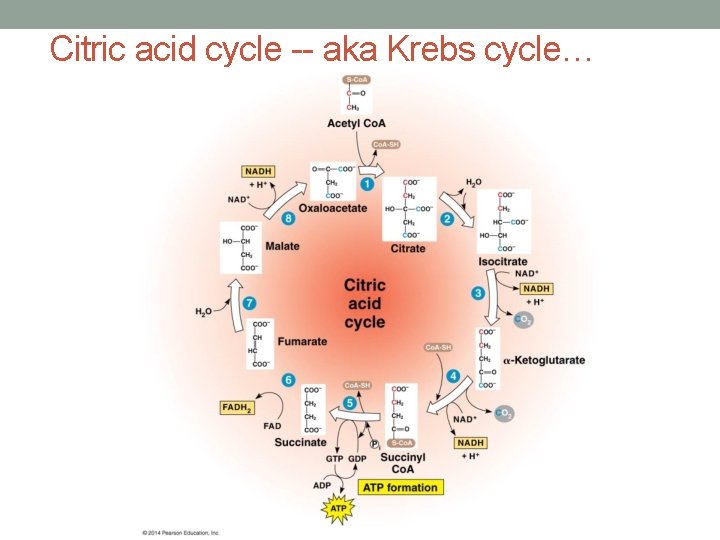
Citric acid cycle -- aka Krebs cycle…
Krebs cycle, the movie
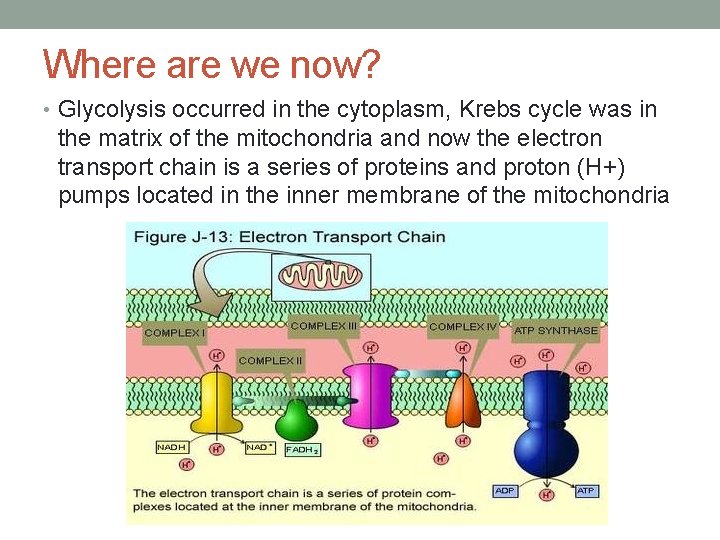
Where are we now? • Glycolysis occurred in the cytoplasm, Krebs cycle was in the matrix of the mitochondria and now the electron transport chain is a series of proteins and proton (H+) pumps located in the inner membrane of the mitochondria
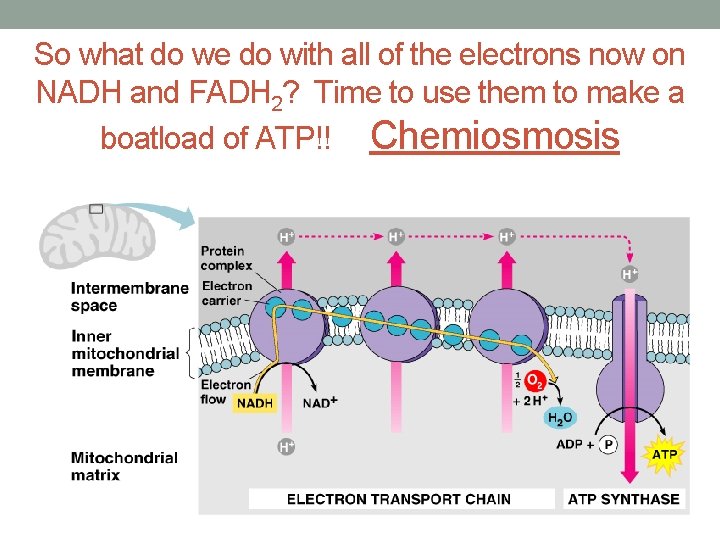
So what do we do with all of the electrons now on NADH and FADH 2? Time to use them to make a boatload of ATP!! Chemiosmosis
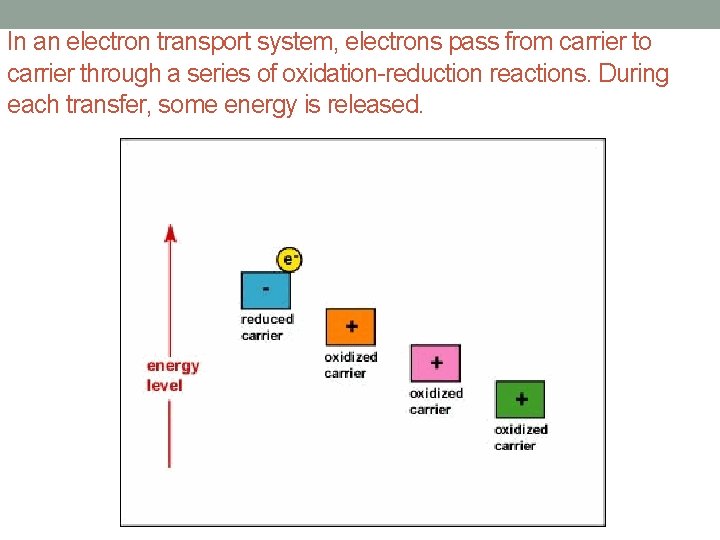
In an electron transport system, electrons pass from carrier to carrier through a series of oxidation-reduction reactions. During each transfer, some energy is released.
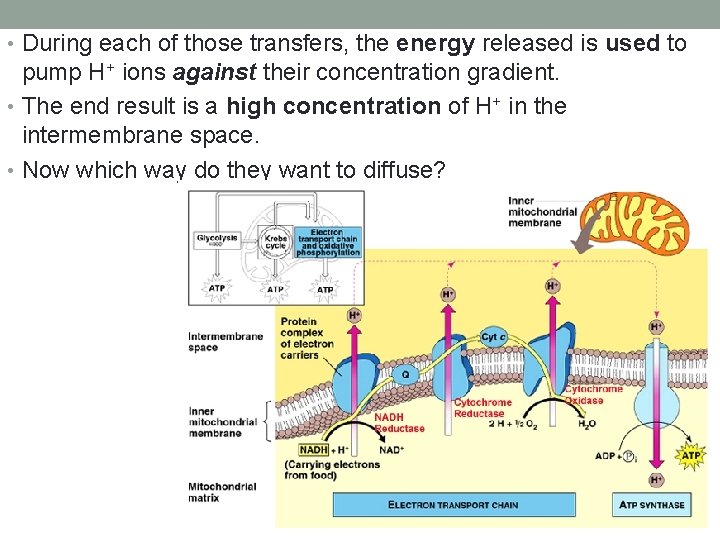
• During each of those transfers, the energy released is used to pump H+ ions against their concentration gradient. • The end result is a high concentration of H+ in the intermembrane space. • Now which way do they want to diffuse?

You know this stuff… • Diffusion of something down its concentration gradient is favorable – AND thus it releases energy • When H+ diffuses back into the matrix through the ATP synthase, it releases energy and this energy drives the production of ATP from ADP and Pi • So here we’re coupling an exergonic (H+ diffusing into the matrix) with an endergonic (making ATP from ADP) reaction
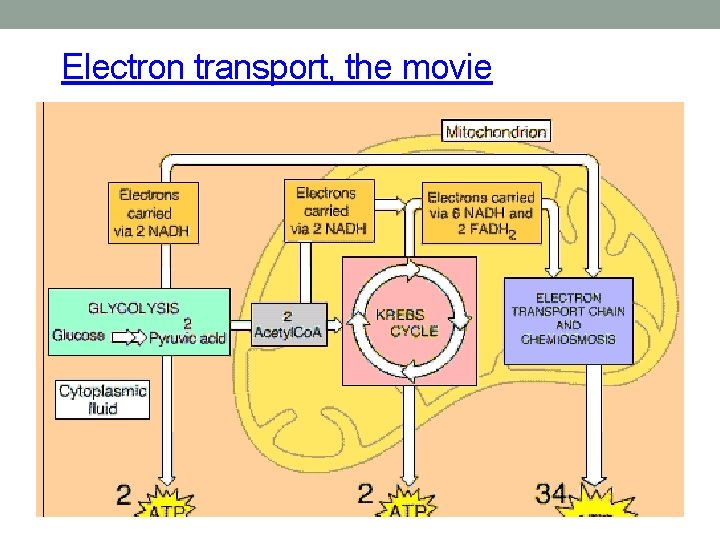
Electron transport, the movie
ETC videos • https: //www. youtube. com/watch? v=xb. J 0 nbzt 5 Kw • https: //www. youtube. com/watch? v=6 W-7 FG 9 Klp. A

ATP synthase (Or F 0/F 1 ATPase) • https: //www. youtube. com/watch? v=3 y 1 d. O 4 n. Na. KY • https: //www. youtube. com/watch? v=Shs 3 l. FU_OFM
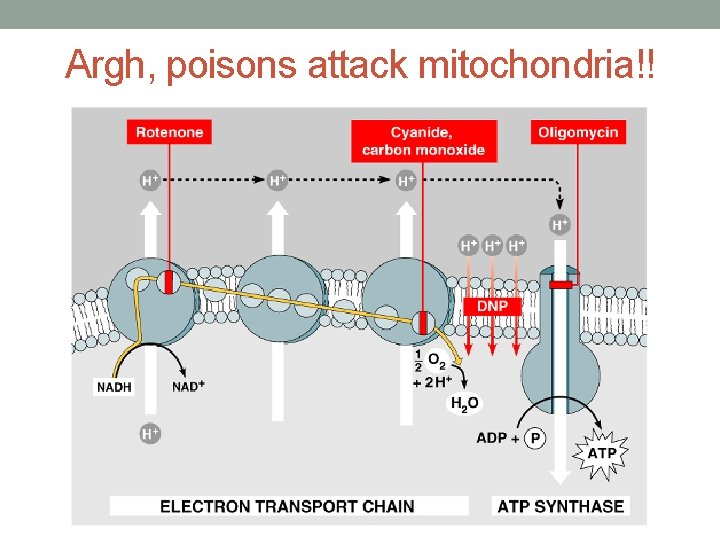
Argh, poisons attack mitochondria!!
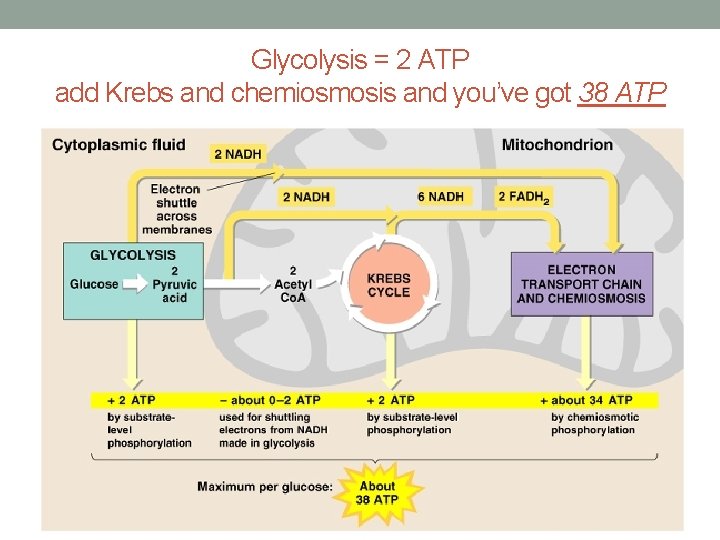
Glycolysis = 2 ATP add Krebs and chemiosmosis and you’ve got 38 ATP
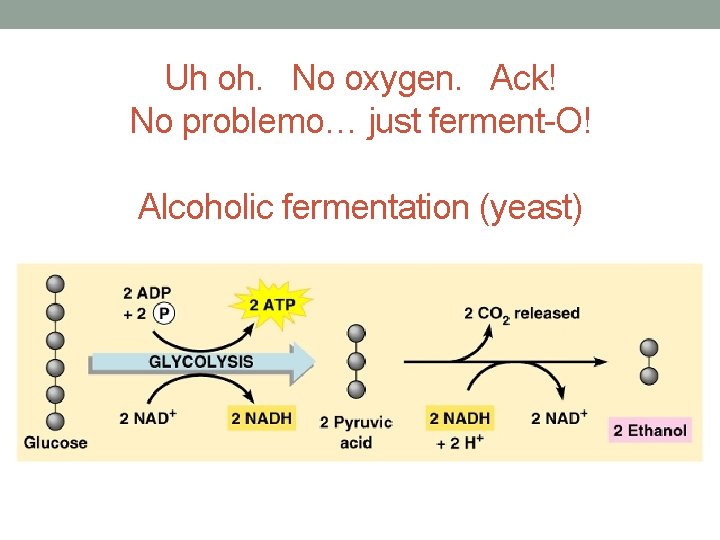
Uh oh. No oxygen. Ack! No problemo… just ferment-O! Alcoholic fermentation (yeast)
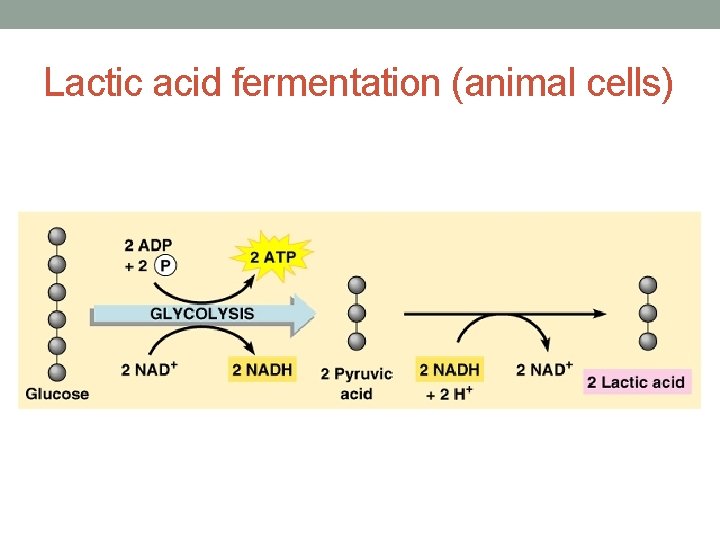
Lactic acid fermentation (animal cells)
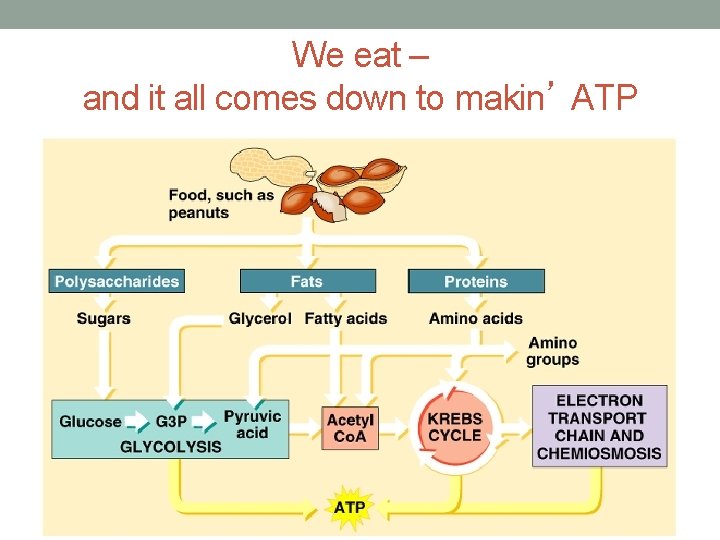
We eat – and it all comes down to makin’ ATP
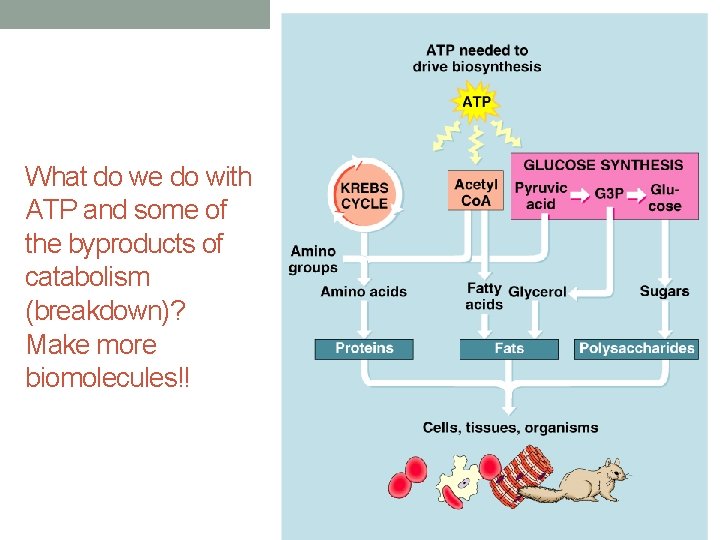
What do we do with ATP and some of the byproducts of catabolism (breakdown)? Make more biomolecules!!
• Crash Course video: https: //www. youtube. com/watch? v=00 jb. G_cf. Gu. Q
- Slides: 43