Cellular mechanisms of signaling evolve from available components
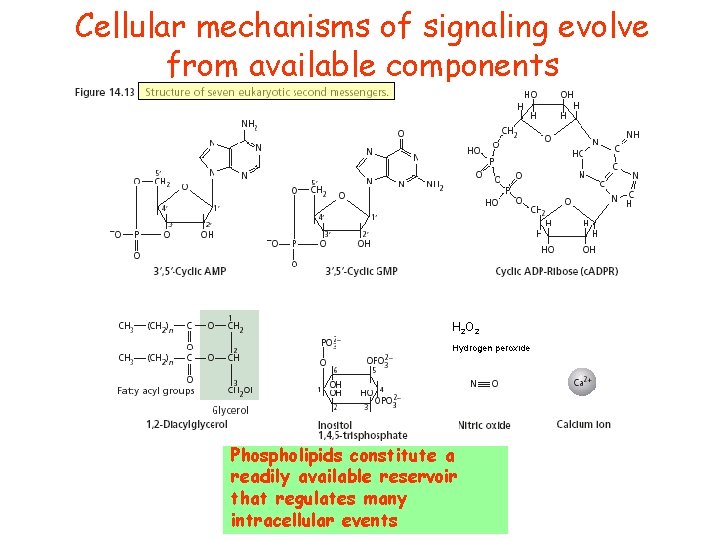
Cellular mechanisms of signaling evolve from available components H 2 O 2 Hydrogen peroxide Phospholipids constitute a readily available reservoir that regulates many intracellular events
Ptd. Ins in yeast
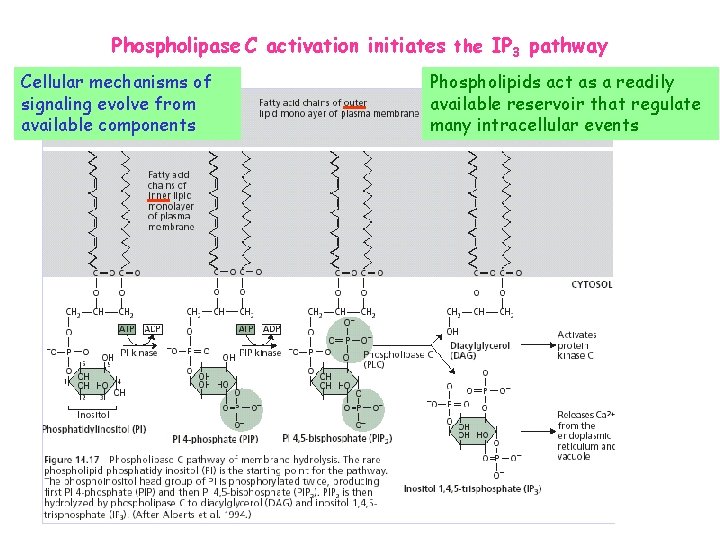
Phospholipase C activation initiates the IP 3 pathway Cellular mechanisms of signaling evolve from available components Phospholipids act as a readily available reservoir that regulate many intracellular events
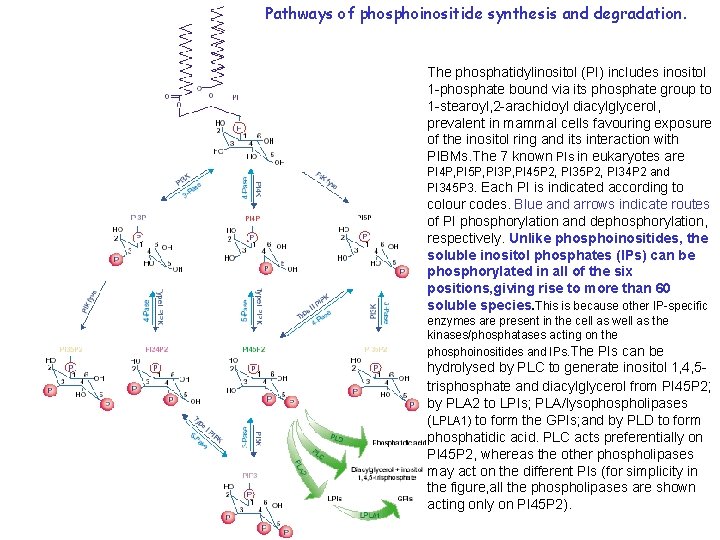
Pathways of phosphoinositide synthesis and degradation. The phosphatidylinositol (PI) includes inositol 1 -phosphate bound via its phosphate group to 1 -stearoyl, 2 -arachidoyl diacylglycerol, prevalent in mammal cells favouring exposure of the inositol ring and its interaction with PIBMs. The 7 known PIs in eukaryotes are PI 4 P, PI 5 P, PI 3 P, PI 45 P 2, PI 34 P 2 and PI 345 P 3. Each PI is indicated according to colour codes. Blue and arrows indicate routes of PI phosphorylation and dephosphorylation, respectively. Unlike phosphoinositides, the soluble inositol phosphates (IPs) can be phosphorylated in all of the six positions, giving rise to more than 60 soluble species. This is because other IP-specific enzymes are present in the cell as well as the kinases/phosphatases acting on the phosphoinositides and IPs. The PIs can be hydrolysed by PLC to generate inositol 1, 4, 5 trisphosphate and diacylglycerol from PI 45 P 2; by PLA 2 to LPIs; PLA/lysophospholipases (LPLA 1) to form the GPIs; and by PLD to form phosphatidic acid. PLC acts preferentially on PI 45 P 2, whereas the other phospholipases may act on the different PIs (for simplicity in the figure, all the phospholipases are shown acting only on PI 45 P 2).
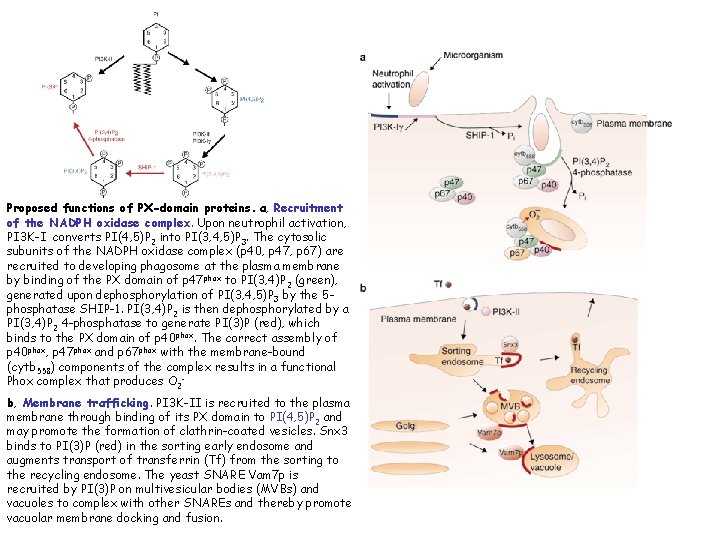
Proposed functions of PX-domain proteins. a, Recruitment of the NADPH oxidase complex. Upon neutrophil activation, PI 3 K-I converts PI(4, 5)P 2 into PI(3, 4, 5)P 3. The cytosolic subunits of the NADPH oxidase complex (p 40, p 47, p 67) are recruited to developing phagosome at the plasma membrane by binding of the PX domain of p 47 phox to PI(3, 4)P 2 (green), generated upon dephosphorylation of PI(3, 4, 5)P 3 by the 5 phosphatase SHIP-1. PI(3, 4)P 2 is then dephosphorylated by a PI(3, 4)P 2 4 -phosphatase to generate PI(3)P (red), which binds to the PX domain of p 40 phox. The correct assembly of p 40 phox, p 47 phox and p 67 phox with the membrane-bound (cytb 558) components of the complex results in a functional Phox complex that produces O 2 b, Membrane trafficking. PI 3 K-II is recruited to the plasma membrane through binding of its PX domain to PI(4, 5)P 2 and may promote the formation of clathrin-coated vesicles. Snx 3 binds to PI(3)P (red) in the sorting early endosome and augments transport of transferrin (Tf) from the sorting to the recycling endosome. The yeast SNARE Vam 7 p is recruited by PI(3)P on multivesicular bodies (MVBs) and vacuoles to complex with other SNAREs and thereby promote vacuolar membrane docking and fusion.
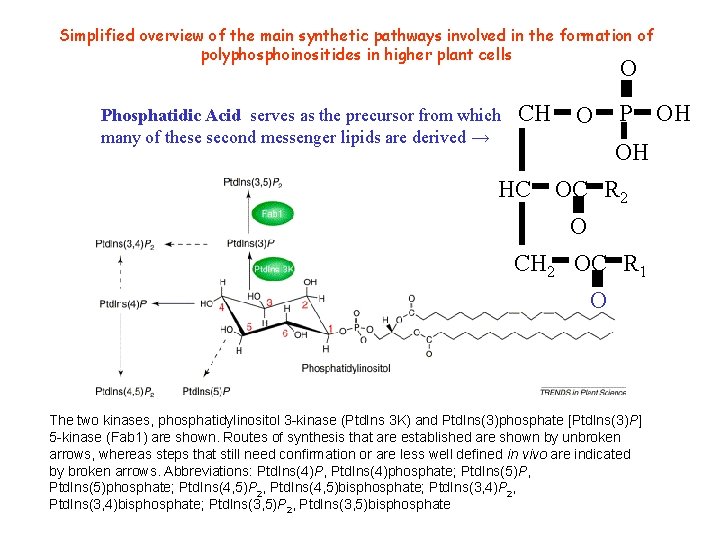
Simplified overview of the main synthetic pathways involved in the formation of polyphosphoinositides in higher plant cells O Phosphatidic Acid serves as the precursor from which CH many of these second messenger lipids are derived → 2 HC O P OH OH OC R 2 O CH 2 OC R 1 O The two kinases, phosphatidylinositol 3 -kinase (Ptd. Ins 3 K) and Ptd. Ins(3)phosphate [Ptd. Ins(3)P] 5 -kinase (Fab 1) are shown. Routes of synthesis that are established are shown by unbroken arrows, whereas steps that still need confirmation or are less well defined in vivo are indicated by broken arrows. Abbreviations: Ptd. Ins(4)P, Ptd. Ins(4)phosphate; Ptd. Ins(5)P, Ptd. Ins(5)phosphate; Ptd. Ins(4, 5)P 2, Ptd. Ins(4, 5)bisphosphate; Ptd. Ins(3, 4)P 2, Ptd. Ins(3, 4)bisphosphate; Ptd. Ins(3, 5)P 2, Ptd. Ins(3, 5)bisphosphate
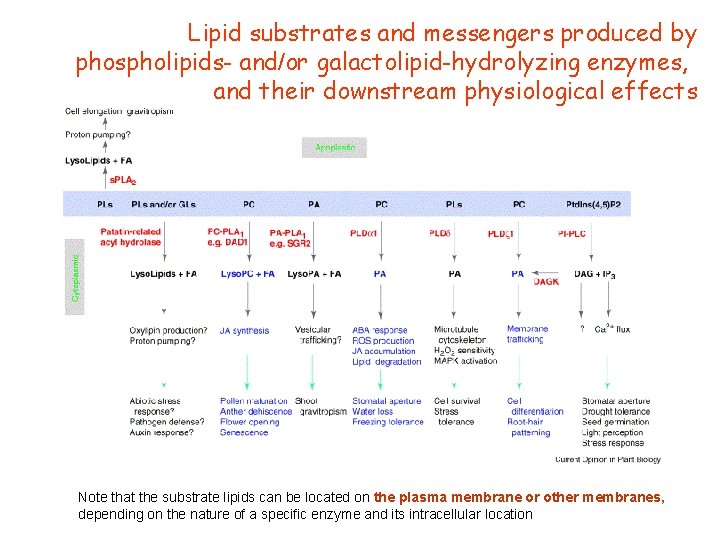
Lipid substrates and messengers produced by phospholipids- and/or galactolipid-hydrolyzing enzymes, and their downstream physiological effects Note that the substrate lipids can be located on the plasma membrane or other membranes, depending on the nature of a specific enzyme and its intracellular location
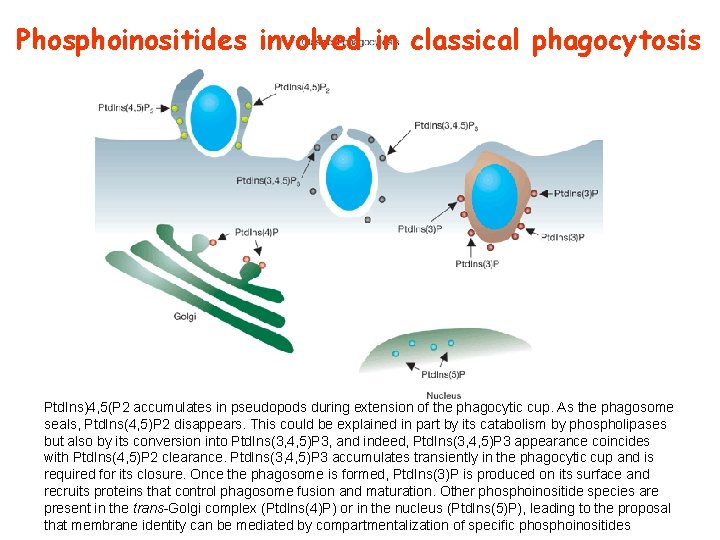
Phosphoinositides involved in classical phagocytosis Ptd. Ins)4, 5(P 2 accumulates in pseudopods during extension of the phagocytic cup. As the phagosome seals, Ptd. Ins(4, 5)P 2 disappears. This could be explained in part by its catabolism by phospholipases but also by its conversion into Ptd. Ins(3, 4, 5)P 3, and indeed, Ptd. Ins(3, 4, 5)P 3 appearance coincides with Ptd. Ins(4, 5)P 2 clearance. Ptd. Ins(3, 4, 5)P 3 accumulates transiently in the phagocytic cup and is required for its closure. Once the phagosome is formed, Ptd. Ins(3)P is produced on its surface and recruits proteins that control phagosome fusion and maturation. Other phosphoinositide species are present in the trans-Golgi complex (Ptd. Ins(4)P) or in the nucleus (Ptd. Ins(5)P), leading to the proposal that membrane identity can be mediated by compartmentalization of specific phosphoinositides
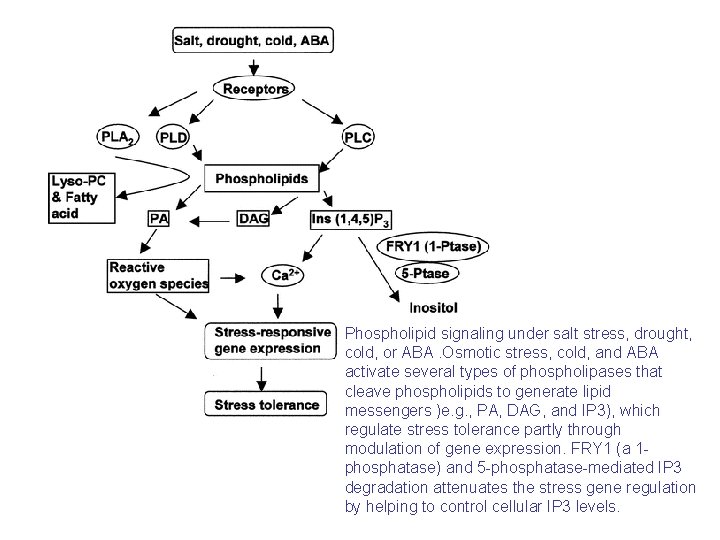
Phospholipid signaling under salt stress, drought, cold, or ABA. Osmotic stress, cold, and ABA activate several types of phospholipases that cleave phospholipids to generate lipid messengers )e. g. , PA, DAG, and IP 3), which regulate stress tolerance partly through modulation of gene expression. FRY 1 (a 1 phosphatase) and 5 -phosphatase-mediated IP 3 degradation attenuates the stress gene regulation by helping to control cellular IP 3 levels.
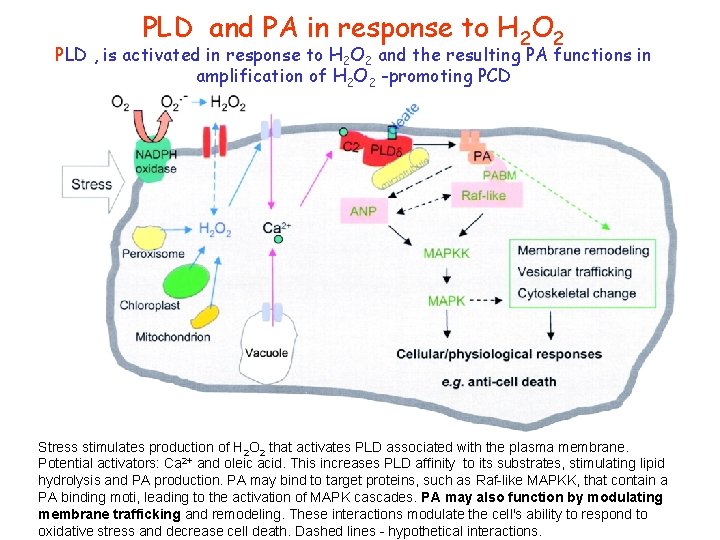
PLD and PA in response to H 2 O 2 PLD , is activated in response to H 2 O 2 and the resulting PA functions in amplification of H 2 O 2 -promoting PCD Stress stimulates production of H 2 O 2 that activates PLD associated with the plasma membrane. Potential activators: Ca 2+ and oleic acid. This increases PLD affinity to its substrates, stimulating lipid hydrolysis and PA production. PA may bind to target proteins, such as Raf-like MAPKK, that contain a PA binding moti, leading to the activation of MAPK cascades. PA may also function by modulating membrane trafficking and remodeling. These interactions modulate the cell's ability to respond to oxidative stress and decrease cell death. Dashed lines - hypothetical interactions.
PLD & PA • Knockout of PLD renders Arabidopsis plants more sensitive to the reactive oxygen species H 2 O 2 and to stresses • H 2 O 2 activates PLD , and PLD -derived PA functions to decrease the promotion of cell death by H 2 O 2. These results suggest that both PLD and its product PA play a positive role in signaling stress responses • PLD and its derivative PA provide a link between phospholipid signaling and H 2 O 2 -promoted cell death. PLD and PA positively regulate plant cell survival and stress responses.
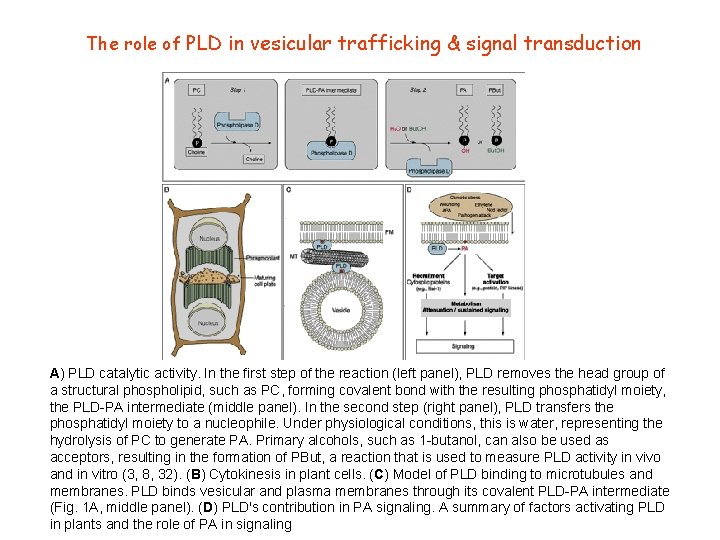
The role of PLD in vesicular trafficking & signal transduction A) PLD catalytic activity. In the first step of the reaction (left panel), PLD removes the head group of a structural phospholipid, such as PC, forming covalent bond with the resulting phosphatidyl moiety, the PLD-PA intermediate (middle panel). In the second step (right panel), PLD transfers the phosphatidyl moiety to a nucleophile. Under physiological conditions, this is water, representing the hydrolysis of PC to generate PA. Primary alcohols, such as 1 -butanol, can also be used as acceptors, resulting in the formation of PBut, a reaction that is used to measure PLD activity in vivo and in vitro (3, 8, 32). (B) Cytokinesis in plant cells. (C) Model of PLD binding to microtubules and membranes. PLD binds vesicular and plasma membranes through its covalent PLD-PA intermediate (Fig. 1 A, middle panel). (D) PLD's contribution in PA signaling. A summary of factors activating PLD in plants and the role of PA in signaling
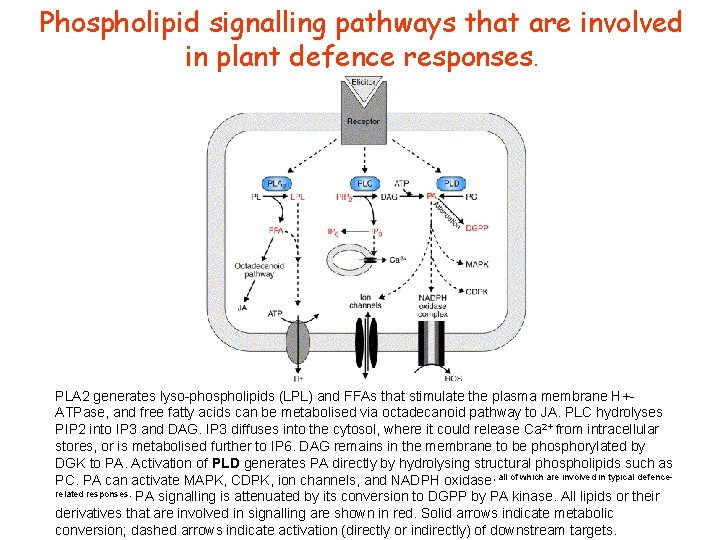
Phospholipid signalling pathways that are involved in plant defence responses. PLA 2 generates lyso-phospholipids (LPL) and FFAs that stimulate the plasma membrane H+ATPase, and free fatty acids can be metabolised via octadecanoid pathway to JA. PLC hydrolyses PIP 2 into IP 3 and DAG. IP 3 diffuses into the cytosol, where it could release Ca 2+ from intracellular stores, or is metabolised further to IP 6. DAG remains in the membrane to be phosphorylated by DGK to PA. Activation of PLD generates PA directly by hydrolysing structural phospholipids such as PC. PA can activate MAPK, CDPK, ion channels, and NADPH oxidase, all of which are involved in typical defencerelated responses. PA signalling is attenuated by its conversion to DGPP by PA kinase. All lipids or their derivatives that are involved in signalling are shown in red. Solid arrows indicate metabolic conversion; dashed arrows indicate activation (directly or indirectly) of downstream targets.
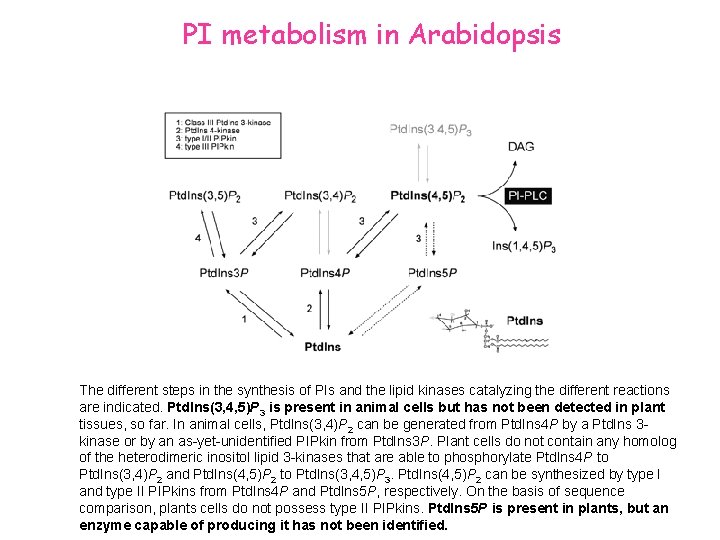
PI metabolism in Arabidopsis The different steps in the synthesis of PIs and the lipid kinases catalyzing the different reactions are indicated. Ptd. Ins(3, 4, 5)P 3 is present in animal cells but has not been detected in plant tissues, so far. In animal cells, Ptd. Ins(3, 4)P 2 can be generated from Ptd. Ins 4 P by a Ptd. Ins 3 kinase or by an as-yet-unidentified PIPkin from Ptd. Ins 3 P. Plant cells do not contain any homolog of the heterodimeric inositol lipid 3 -kinases that are able to phosphorylate Ptd. Ins 4 P to Ptd. Ins(3, 4)P 2 and Ptd. Ins(4, 5)P 2 to Ptd. Ins(3, 4, 5)P 3. Ptd. Ins(4, 5)P 2 can be synthesized by type I and type II PIPkins from Ptd. Ins 4 P and Ptd. Ins 5 P, respectively. On the basis of sequence comparison, plants cells do not possess type II PIPkins. Ptd. Ins 5 P is present in plants, but an enzyme capable of producing it has not been identified.
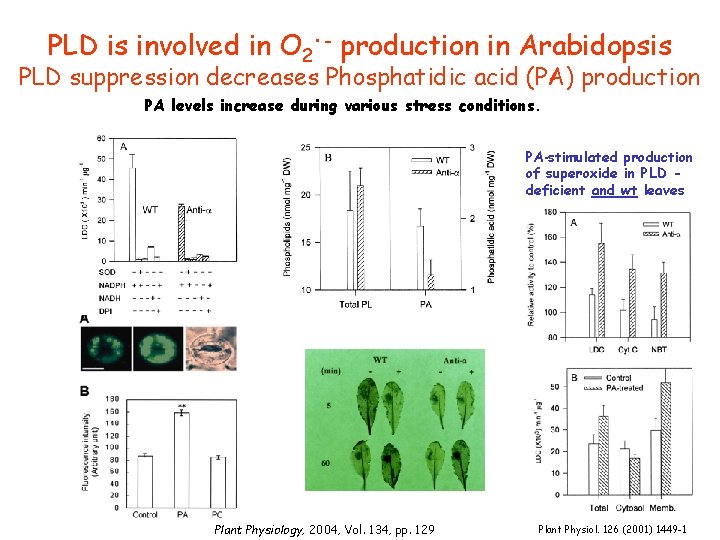
. PLD is involved in O 2 - production in Arabidopsis PLD suppression decreases Phosphatidic acid (PA) production PA levels increase during various stress conditions. PA-stimulated production of superoxide in PLD deficient and wt leaves Plant Physiology, 2004, Vol. 134, pp. 129 Plant Physiol. 126 (2001) 1449 -1
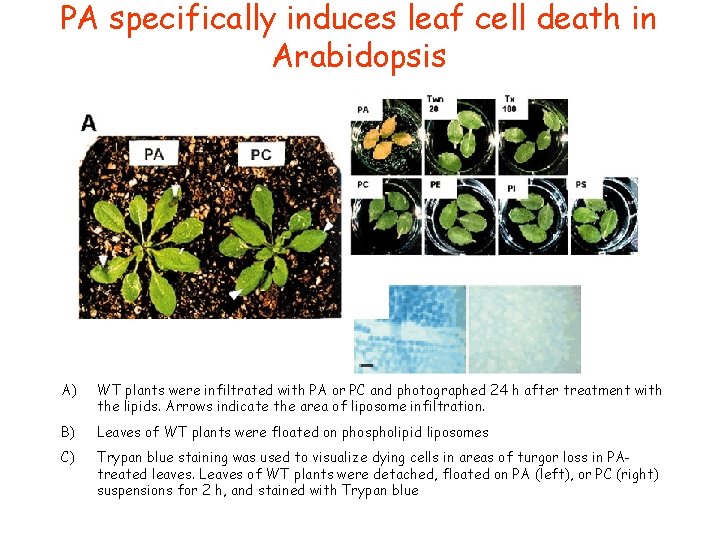
PA specifically induces leaf cell death in Arabidopsis A) WT plants were infiltrated with PA or PC and photographed 24 h after treatment with the lipids. Arrows indicate the area of liposome infiltration. B) Leaves of WT plants were floated on phospholipid liposomes C) Trypan blue staining was used to visualize dying cells in areas of turgor loss in PAtreated leaves. Leaves of WT plants were detached, floated on PA (left), or PC (right) suspensions for 2 h, and stained with Trypan blue
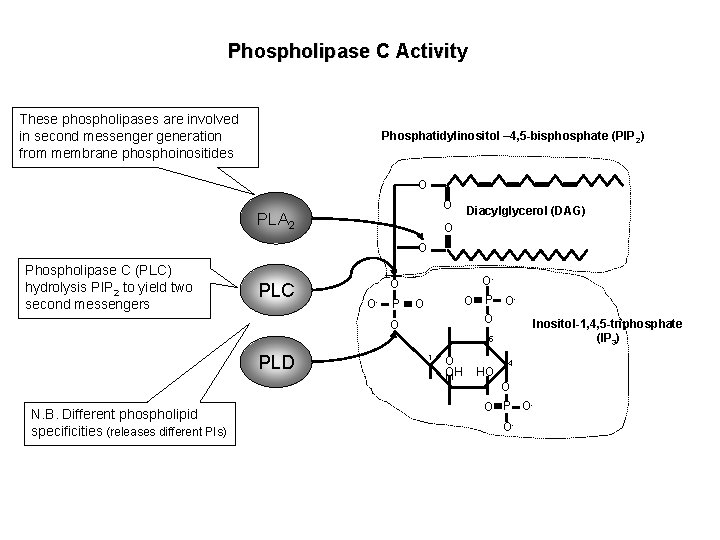
Phospholipase C Activity These phospholipases are involved in second messenger generation from membrane phosphoinositides Phosphatidylinositol – 4, 5 -bisphosphate (PIP 2) O O PLA 2 Diacylglycerol (DAG) O O Phospholipase C (PLC) hydrolysis PIP 2 to yield two second messengers PLC O- O O- P O O O P OO 5 PLD N. B. Different phospholipid specificities (releases different PIs) 1 O OH H HO Inositol-1, 4, 5 -triphosphate (IP 3) 4 O O P OO-
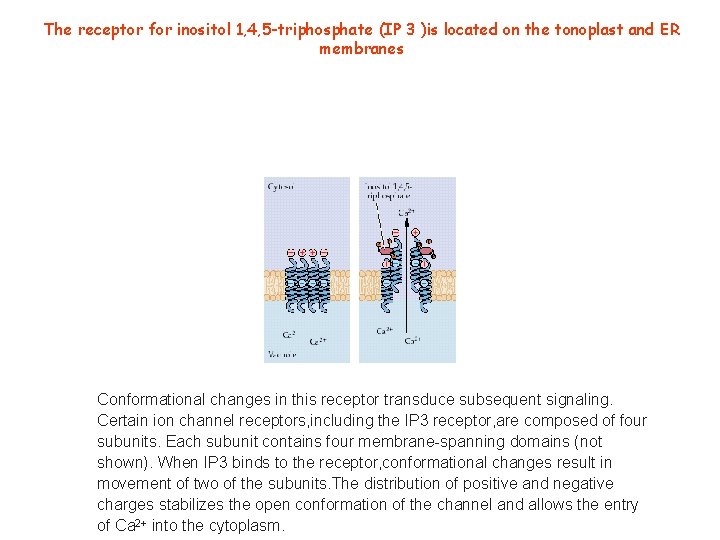
The receptor for inositol 1, 4, 5 -triphosphate (IP 3 )is located on the tonoplast and ER membranes Conformational changes in this receptor transduce subsequent signaling. Certain ion channel receptors, including the IP 3 receptor, are composed of four subunits. Each subunit contains four membrane-spanning domains (not shown). When IP 3 binds to the receptor, conformational changes result in movement of two of the subunits. The distribution of positive and negative charges stabilizes the open conformation of the channel and allows the entry of Ca 2+ into the cytoplasm.
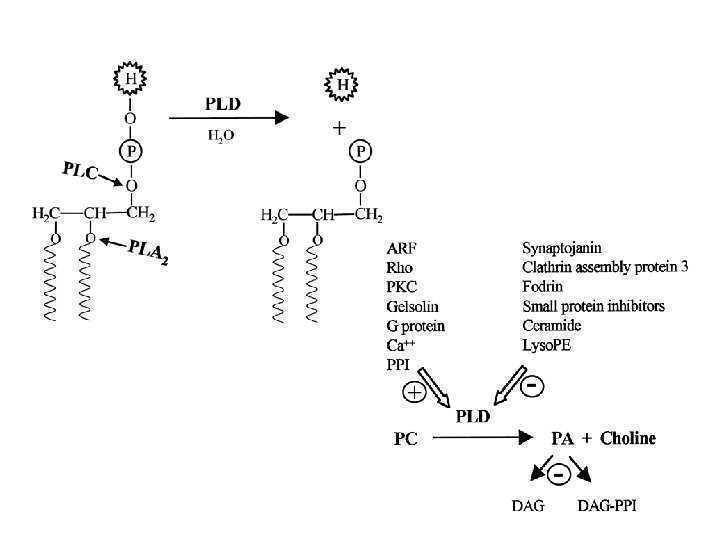
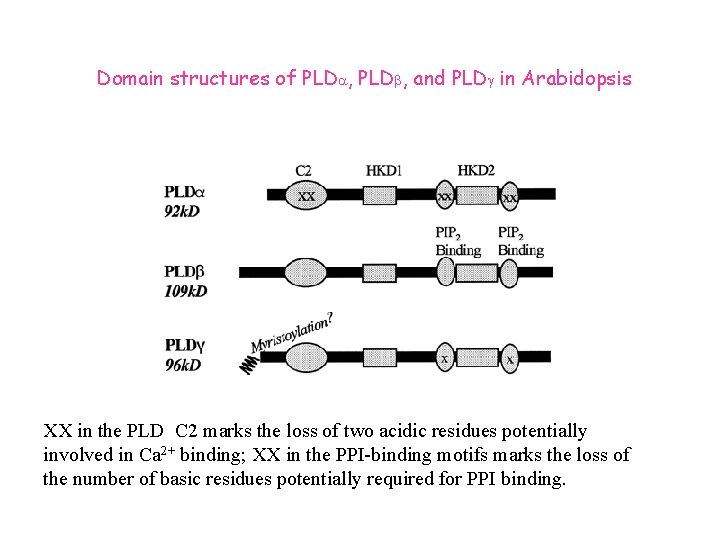
Domain structures of PLDa, PLDb, and PLDg in Arabidopsis XX in the PLD C 2 marks the loss of two acidic residues potentially involved in Ca 2+ binding; XX in the PPI-binding motifs marks the loss of the number of basic residues potentially required for PPI binding.
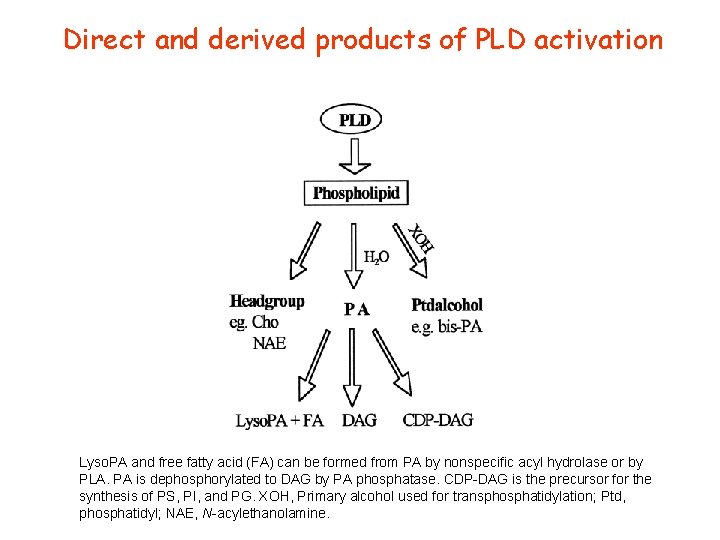
Direct and derived products of PLD activation Lyso. PA and free fatty acid (FA) can be formed from PA by nonspecific acyl hydrolase or by PLA. PA is dephosphorylated to DAG by PA phosphatase. CDP-DAG is the precursor for the synthesis of PS, PI, and PG. XOH, Primary alcohol used for transphosphatidylation; Ptd, phosphatidyl; NAE, N-acylethanolamine.
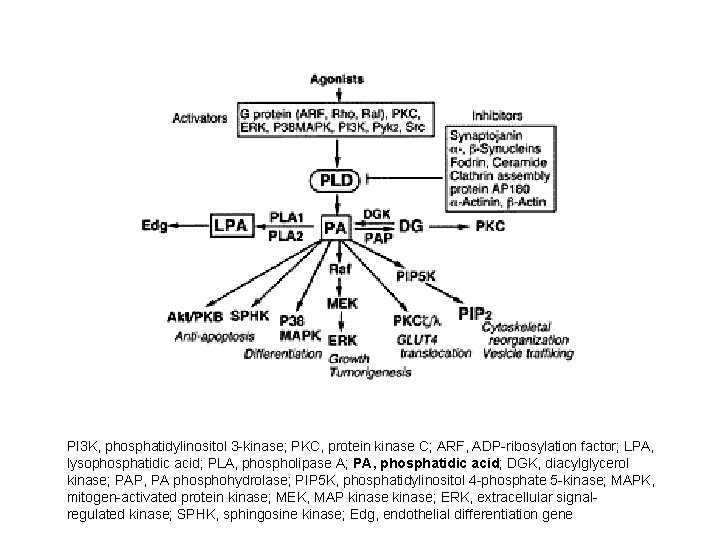
PI 3 K, phosphatidylinositol 3 -kinase; PKC, protein kinase C; ARF, ADP-ribosylation factor; LPA, lysophosphatidic acid; PLA, phospholipase A; PA, phosphatidic acid; DGK, diacylglycerol kinase; PAP, PA phosphohydrolase; PIP 5 K, phosphatidylinositol 4 -phosphate 5 -kinase; MAPK, mitogen-activated protein kinase; MEK, MAP kinase; ERK, extracellular signalregulated kinase; SPHK, sphingosine kinase; Edg, endothelial differentiation gene
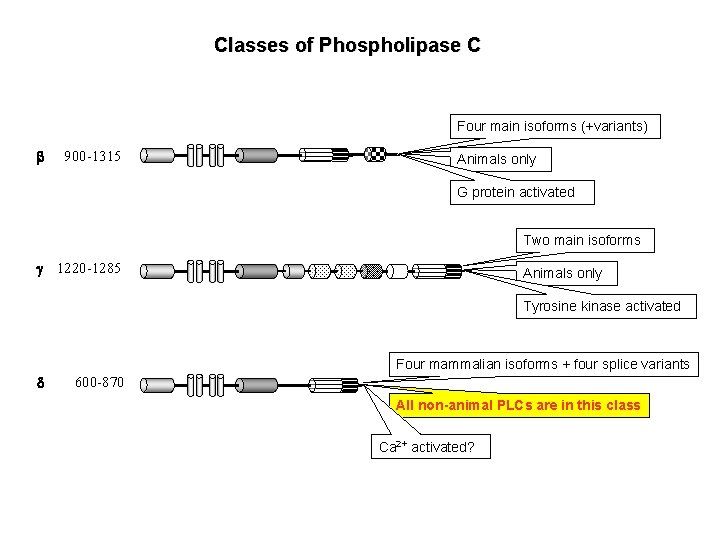
Classes of Phospholipase C Four main isoforms (+variants) b 900 -1315 Animals only G protein activated Two main isoforms g 1220 -1285 Animals only Tyrosine kinase activated Four mammalian isoforms + four splice variants d 600 -870 All non-animal PLCs are in this class Ca 2+ activated?
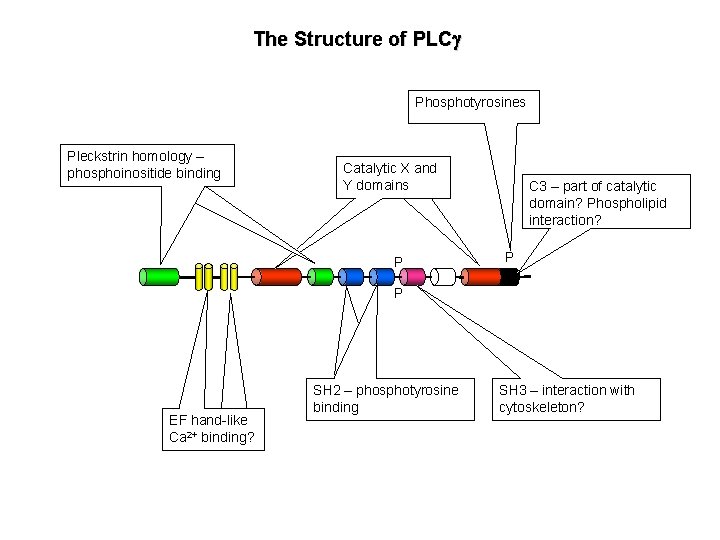
The Structure of PLCg Phosphotyrosines Pleckstrin homology – phosphoinositide binding Catalytic X and Y domains P C 3 – part of catalytic domain? Phospholipid interaction? P P EF hand-like Ca 2+ binding? SH 2 – phosphotyrosine binding SH 3 – interaction with cytoskeleton?
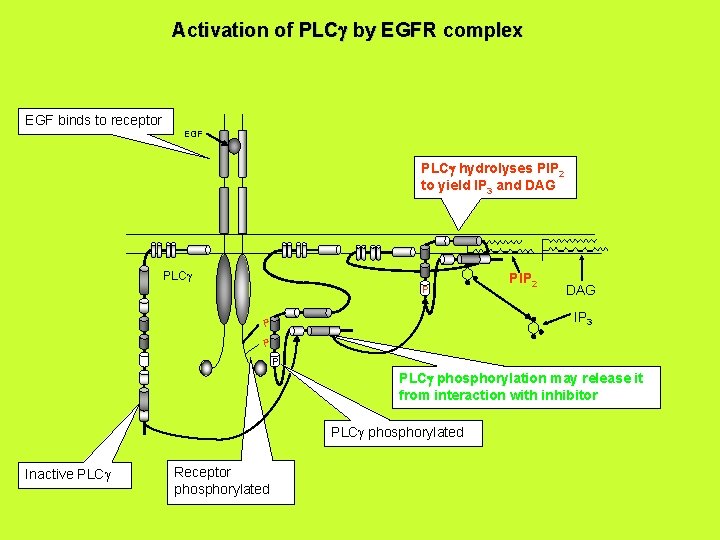
Activation of PLCg by EGFR complex EGF binds to receptor EGF PLCg hydrolyses PIP 2 to yield IP 3 and DAG PLCg P PIP 2 DAG IP 3 P PLCg phosphorylation may release it from interaction with inhibitor PLCg phosphorylated Inactive PLCg Receptor phosphorylated
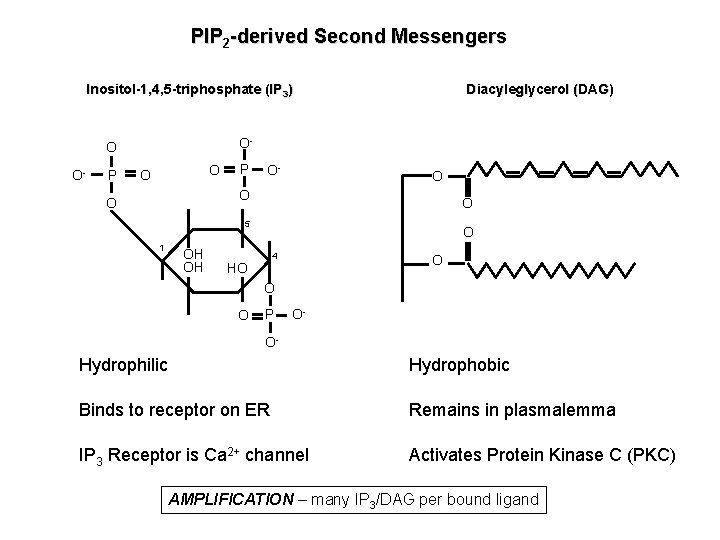
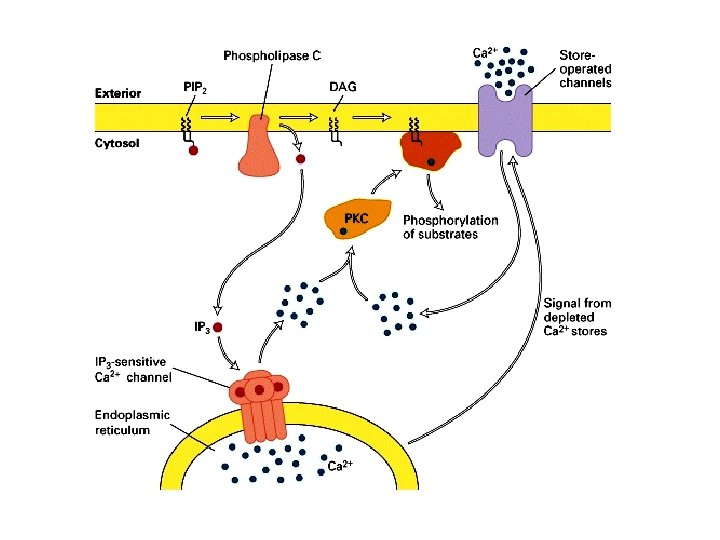
PIP 2 -derived Second Messengers Diacyleglycerol (DAG) Inositol-1, 4, 5 -triphosphate (IP 3) O- O O- P O O P O- O O 5 1 OH OH O 4 HO O P O- O- Hydrophilic Hydrophobic Binds to receptor on ER Remains in plasmalemma IP 3 Receptor is Ca 2+ channel Activates Protein Kinase C (PKC) AMPLIFICATION – many IP 3/DAG per bound ligand
Summary Phosphatidylinositol-specific PLC hydrolyses membrane PIP 2 PLCg has domains that allow binding to phosphotyrosine (SH 2) PLCg associates with activated receptor tyrosine kinases PLCg is activated by tyrosine phosphorylation IP 3 – soluble, induces Ca 2+ release DAG – hydrophobic, activates protein kinase C Loewen, et al (2004). Phospholipid Metabolism Regulated by a Transcription Factor Sensing Phosphatidic Acid. Science 304, 1644 -1647. Inositol-induced alteration in phospholipid synthesis.
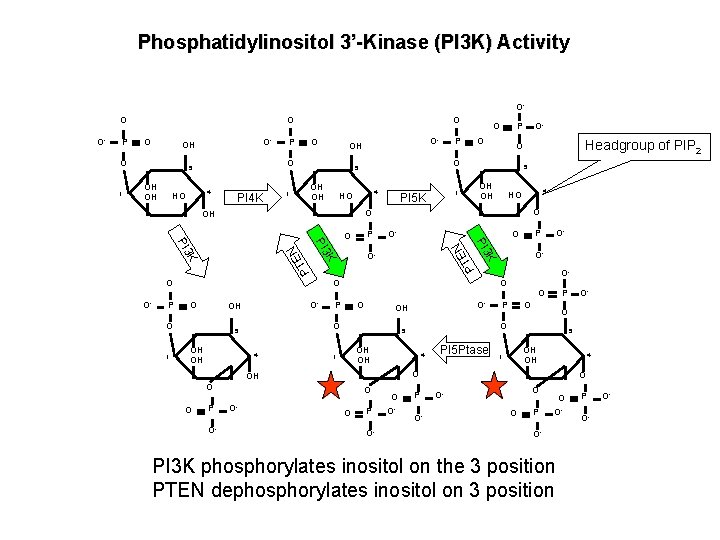
Phosphatidylinositol 3’-Kinase (PI 3 K) Activity OO O 4 HO PI 4 K OH OH 1 4 OH OH 1 PI 5 K P O O- 4 O OH O P P O 4 PI 5 Ptase 5 OH OH 1 4 O O O P O- O O- P O- O- O O OH OH 1 OH O- O- 5 O O- OO P O- O- K K K OH OH P O O- O 5 O 4 HO PI 3 O- OH P Headgroup of PIP 2 O 5 O O 1 O O- O O O P O HO O P P 5 OH O- O- OH O 5 OH OH P EN O 1 O- OH O PT O EN P PT O- O O OO P O O- O- PI 3 K phosphorylates inositol on the 3 position PTEN dephosphorylates inositol on 3 position P O- O-
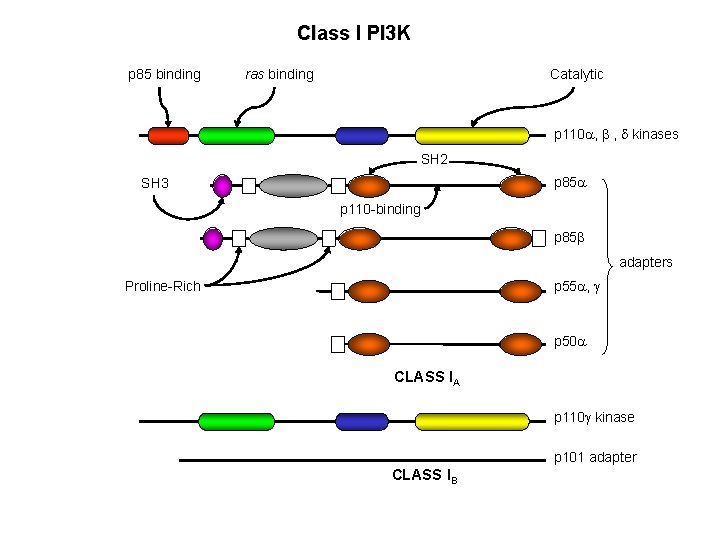
Class I PI 3 K p 85 binding ras binding Catalytic p 110 a, b , d kinases SH 2 p 85 a SH 3 p 110 -binding p 85 b adapters p 55 a, g Proline-Rich p 50 a CLASS IA p 110 g kinase p 101 adapter CLASS IB
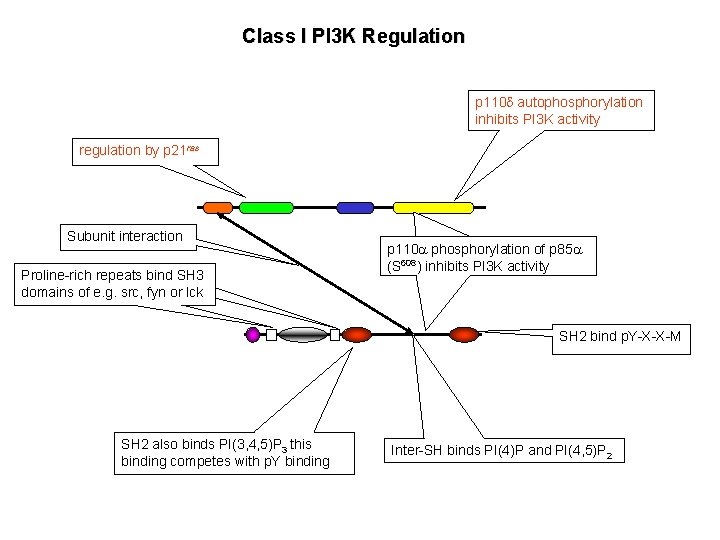
Class I PI 3 K Regulation p 110 d autophosphorylation inhibits PI 3 K activity regulation by p 21 ras Subunit interaction Proline-rich repeats bind SH 3 domains of e. g. src, fyn or lck p 110 a phosphorylation of p 85 a (S 608) inhibits PI 3 K activity SH 2 bind p. Y-X-X-M SH 2 also binds PI(3, 4, 5)P 3 this binding competes with p. Y binding Inter-SH binds PI(4)P and PI(4, 5)P 2
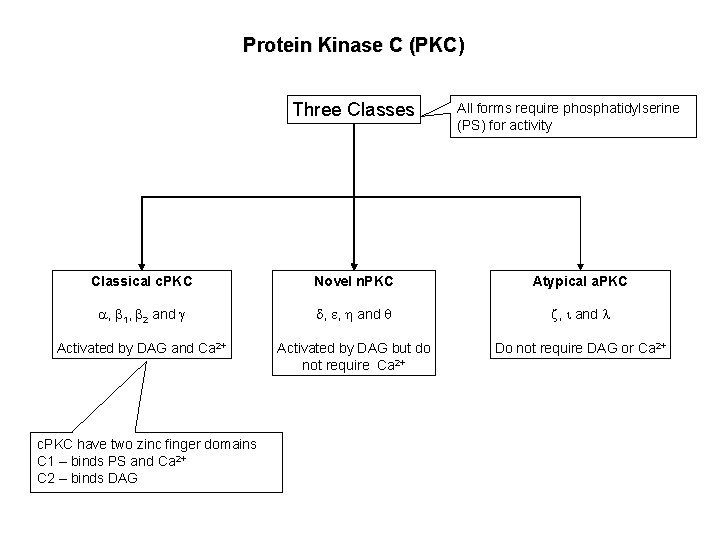
Protein Kinase C (PKC) Three Classes All forms require phosphatidylserine (PS) for activity Classical c. PKC Novel n. PKC Atypical a. PKC a, b 1, b 2 and g d, e, and z, i and l Activated by DAG and Ca 2+ Activated by DAG but do not require Ca 2+ Do not require DAG or Ca 2+ c. PKC have two zinc finger domains C 1 – binds PS and Ca 2+ C 2 – binds DAG
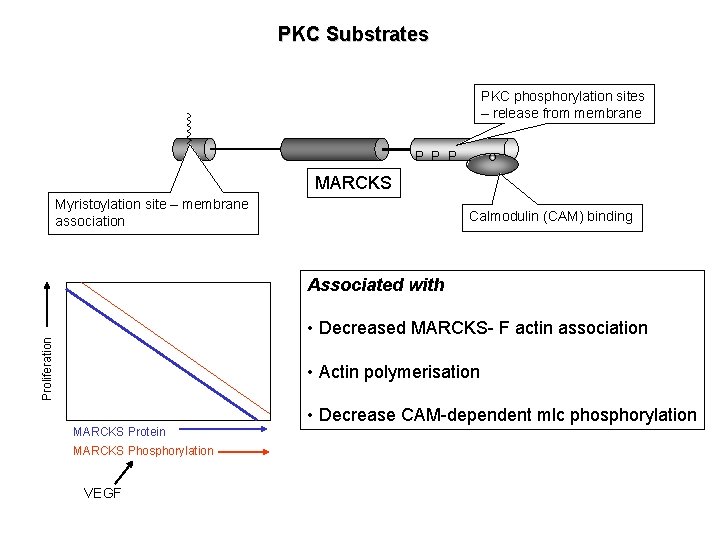
PKC Substrates PKC phosphorylation sites – release from membrane P P P MARCKS Myristoylation site – membrane association Calmodulin (CAM) binding Associated with Proliferation • Decreased MARCKS- F actin association • Actin polymerisation • Decrease CAM-dependent mlc phosphorylation MARCKS Protein MARCKS Phosphorylation VEGF
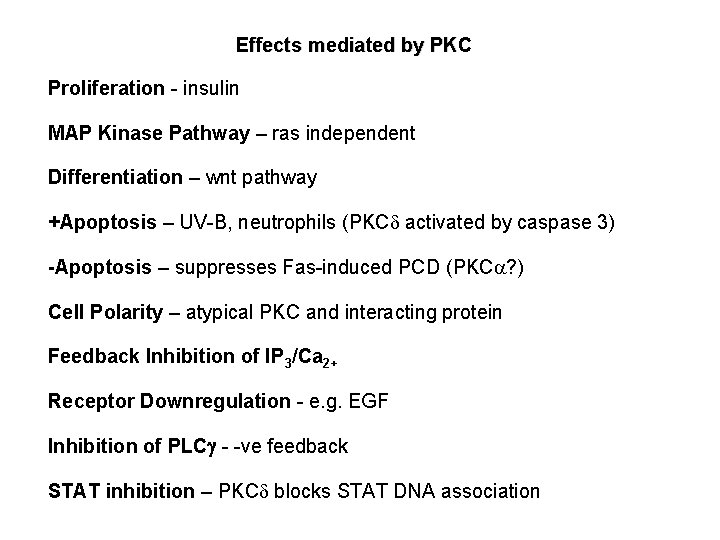
Effects mediated by PKC Proliferation - insulin MAP Kinase Pathway – ras independent Differentiation – wnt pathway +Apoptosis – UV-B, neutrophils (PKCd activated by caspase 3) -Apoptosis – suppresses Fas-induced PCD (PKCa? ) Cell Polarity – atypical PKC and interacting protein Feedback Inhibition of IP 3/Ca 2+ Receptor Downregulation - e. g. EGF Inhibition of PLCg - -ve feedback STAT inhibition – PKCd blocks STAT DNA association
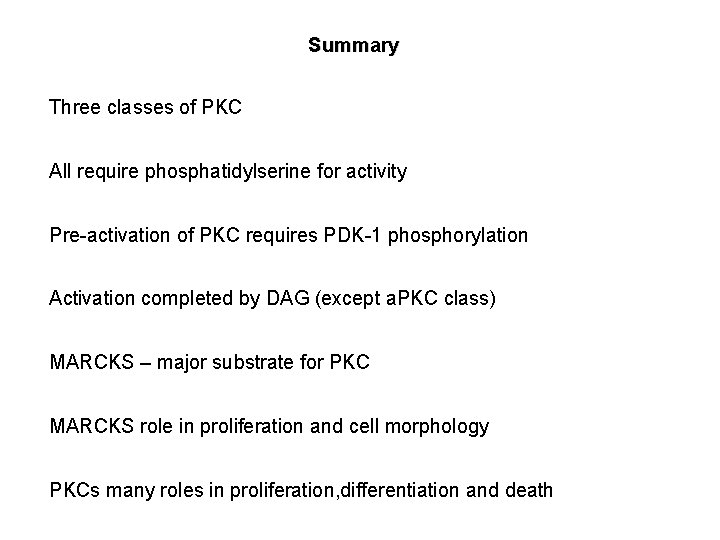
Summary Three classes of PKC All require phosphatidylserine for activity Pre-activation of PKC requires PDK-1 phosphorylation Activation completed by DAG (except a. PKC class) MARCKS – major substrate for PKC MARCKS role in proliferation and cell morphology PKCs many roles in proliferation, differentiation and death
Summary PI 3 kinases phosphorylate phosphoinositides at position 3 PTEN dephosphorylates phosphoinositides at position 3 p 110 contains catalytic activity p 85 responsible for recruiting enzyme to RTK PI-3, 4, 5 -P 3 recruits PDK 1, PDK 2 and Akt PI-3, 4, 5 -P 3 recruits other proteins and regulates cytoskeleton and transport
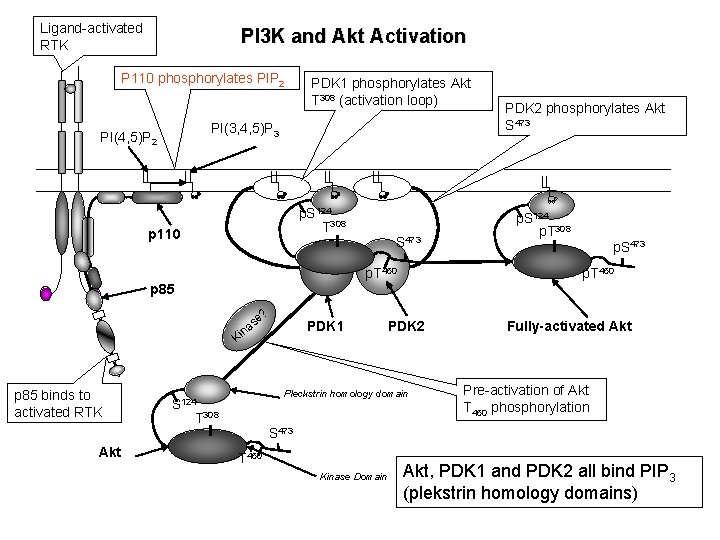
Ligand-activated RTK PI 3 K and Akt Activation P 110 phosphorylates PIP 2 PDK 1 phosphorylates Akt T 308 (activation loop) PI(3, 4, 5)P 3 PI(4, 5)P 2 p. S 124 T 308 p 110 p. T 450 p 85 ? se a n Ki p 85 binds to activated RTK Akt S 473 PDK 1 T 308 p. S 124 p. T 308 p. S 473 p. T 450 PDK 2 Pleckstrin homology domain S 124 PDK 2 phosphorylates Akt S 473 Fully-activated Akt Pre-activation of Akt T 450 phosphorylation S 473 T 450 Kinase Domain Akt, PDK 1 and PDK 2 all bind PIP 3 (plekstrin homology domains)
Other PI-3, 4, 5 -P 3 Functions Regulation of Vesicle Transport either… • Binding to FYVE domain proteins • Regulating small GTP-binding protein Arf Rearrangement of actin cytoskeleton (rac) Recruitment of Tyrosine kinases – PH domains in Btk Enhancement of PLCg – direct interaction
- Slides: 39