Cellular Energy The First Law of Thermodynamics first





- Slides: 13

Cellular Energy

The First Law of Thermodynamics • first law of thermodynamics – Energy can be transferred and transformed, but it cannot be created or destroyed Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

The Second Law of Thermodynamics • second law of thermodynamics: – Every energy transfer or transformation increases the entropy (disorder) of the universe During every energy transfer or transformation, some energy is unusable, and is often lost as heat Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

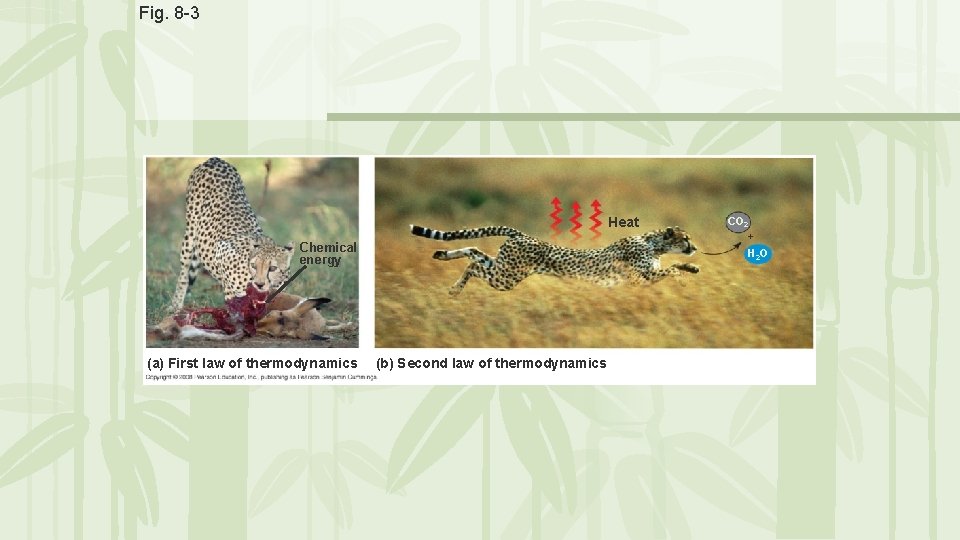
Fig. 8 -3 Heat Chemical energy (a) First law of thermodynamics CO 2 + H 2 O (b) Second law of thermodynamics

Cell Energy • Energy is essential to life • All living organisms must be able to produce energy, store energy and use energy • Cells need a quick source of energy • Cellular energy is made available in chemical bonds of the ATP molecule

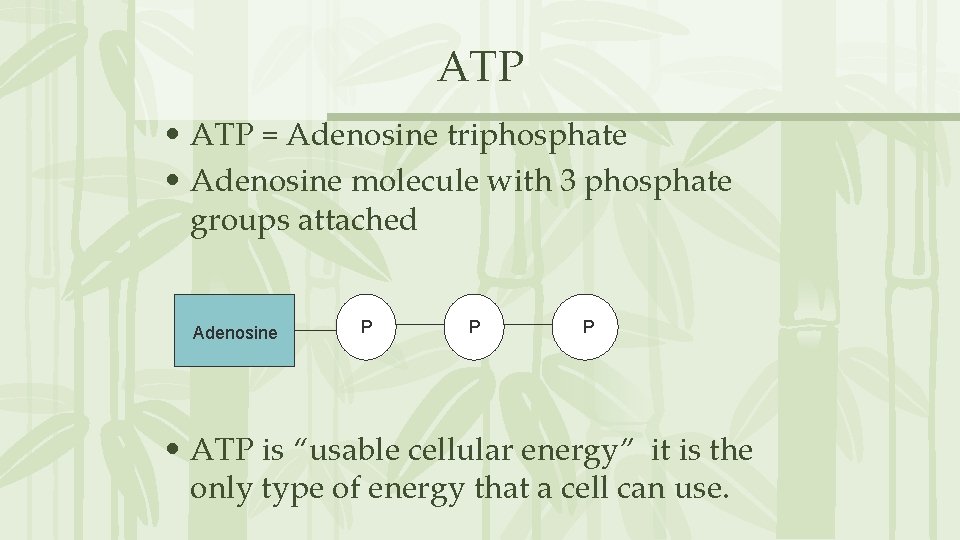
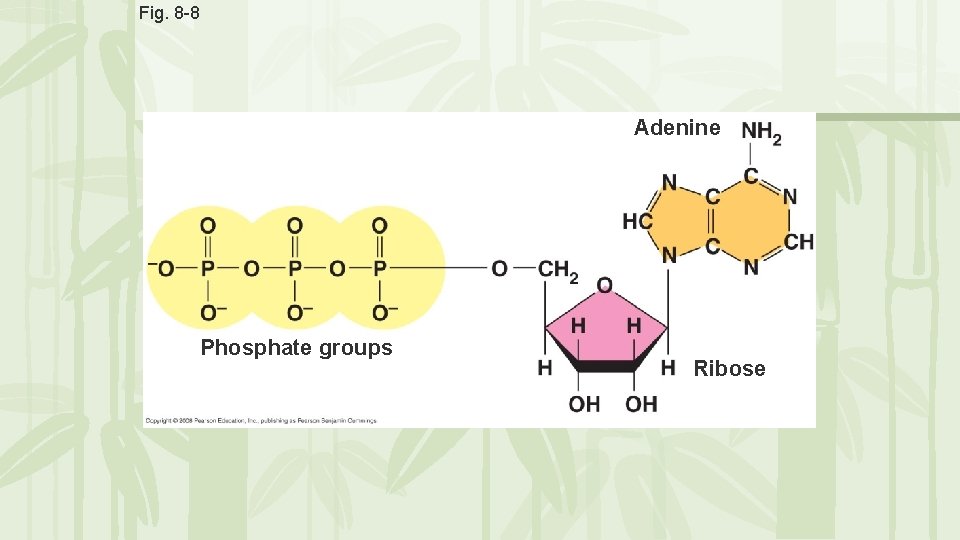
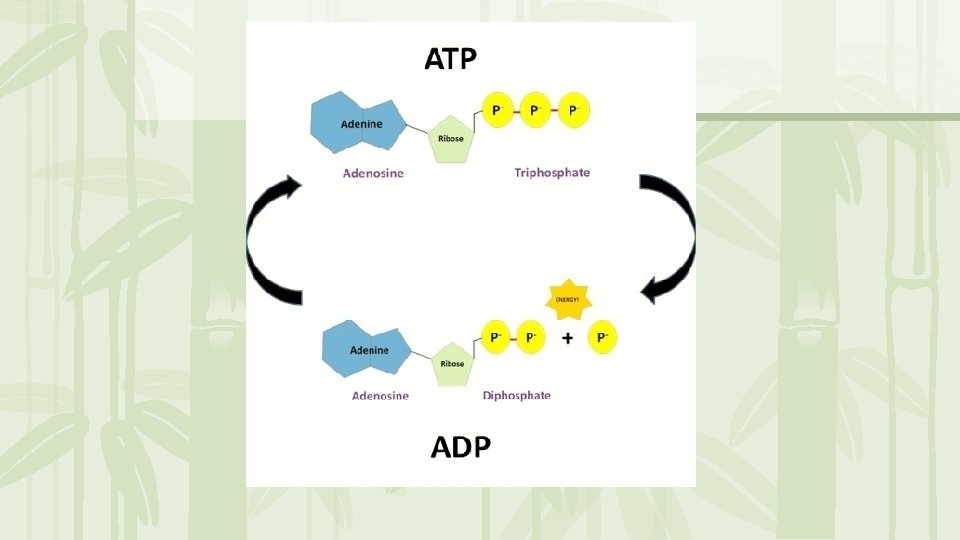
ATP • ATP = Adenosine triphosphate • Adenosine molecule with 3 phosphate groups attached Adenosine P P P • ATP is “usable cellular energy” it is the only type of energy that a cell can use.

Fig. 8 -8 Adenine Phosphate groups Ribose

ATP • Energy is contained in the bond that holds the phosphate molecules to the adenosine • When a bond breaks, energy is released resulting in ADP (adenosine diphosphate)


• Catabolic pathways release energy by breaking down complex molecules into simpler compounds – Cellular respiration, the breakdown of glucose in the presence of oxygen, is an example of a pathway of catabolism Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

• Anabolic pathways consume energy to build complex molecules from simpler ones – The synthesis of protein from amino acids is an example of anabolism Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

An organism’s metabolism transforms matter and energy, subject to the laws of thermodynamics • Metabolism is the totality of an organism’s chemical reactions Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

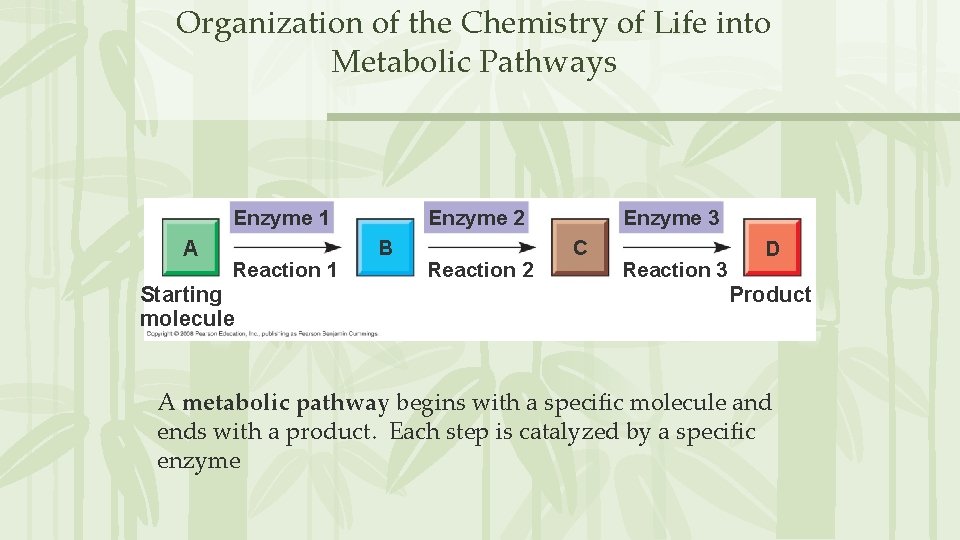
Organization of the Chemistry of Life into Metabolic Pathways Enzyme 1 A Reaction 1 Starting molecule Enzyme 2 B Reaction 2 Enzyme 3 C Reaction 3 D Product A metabolic pathway begins with a specific molecule and ends with a product. Each step is catalyzed by a specific enzyme