CELLULAR ENERGY CHEMICAL DIRECTIVES Entropy Disorder FoodEnergy Stored





![• Cytoplasmic [NADPH] > > [NADP+] • Cytoplasmic [NADH] < < [NAD+] • Cytoplasmic [NADPH] > > [NADP+] • Cytoplasmic [NADH] < < [NAD+]](https://slidetodoc.com/presentation_image_h/0ec840fdb4703dbd124edebeabc4a555/image-18.jpg)





![Potential energy At standard conditions, when [reactant] = [product], DGo = X; X tells Potential energy At standard conditions, when [reactant] = [product], DGo = X; X tells](https://slidetodoc.com/presentation_image_h/0ec840fdb4703dbd124edebeabc4a555/image-26.jpg)
![DG < 0 • Without intervention, as Y X reaction continues, [Y] decreases and DG < 0 • Without intervention, as Y X reaction continues, [Y] decreases and](https://slidetodoc.com/presentation_image_h/0ec840fdb4703dbd124edebeabc4a555/image-27.jpg)


![[Product] [Reactant] when at equilibrium = the Equilibrium Constant (K) [Product] [Reactant] when at equilibrium = the Equilibrium Constant (K)](https://slidetodoc.com/presentation_image_h/0ec840fdb4703dbd124edebeabc4a555/image-31.jpg)









- Slides: 44

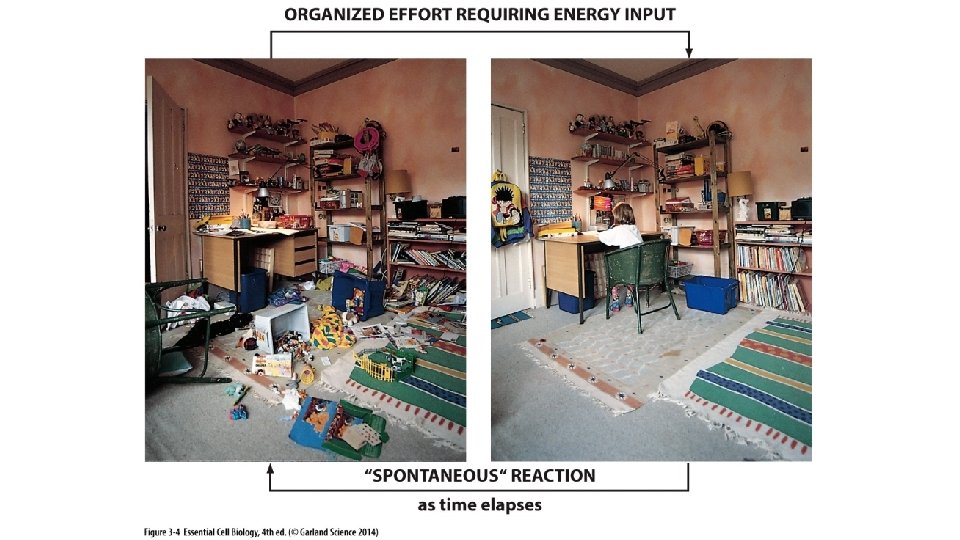
CELLULAR ENERGY & CHEMICAL DIRECTIVES
Entropy




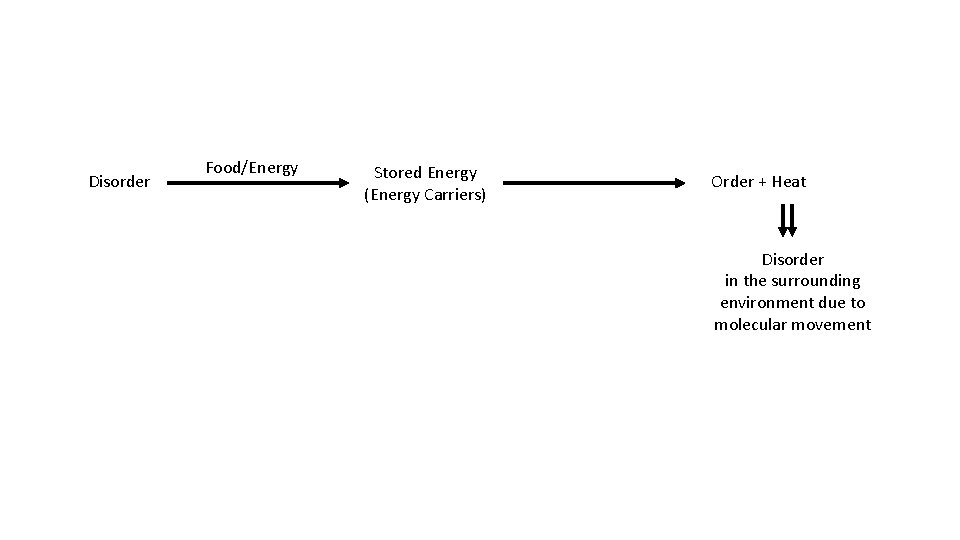
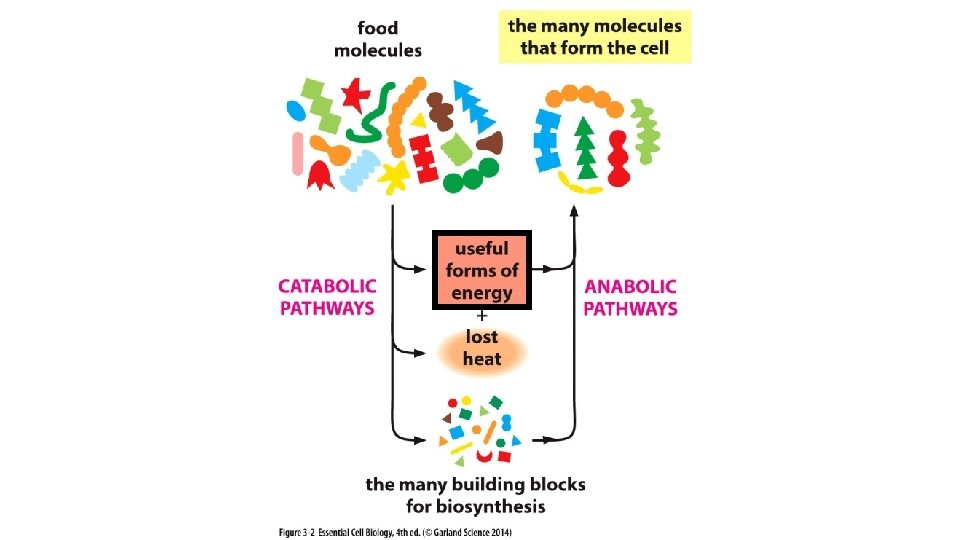
Disorder Food/Energy Stored Energy (Energy Carriers) Order + Heat Disorder in the surrounding environment due to molecular movement

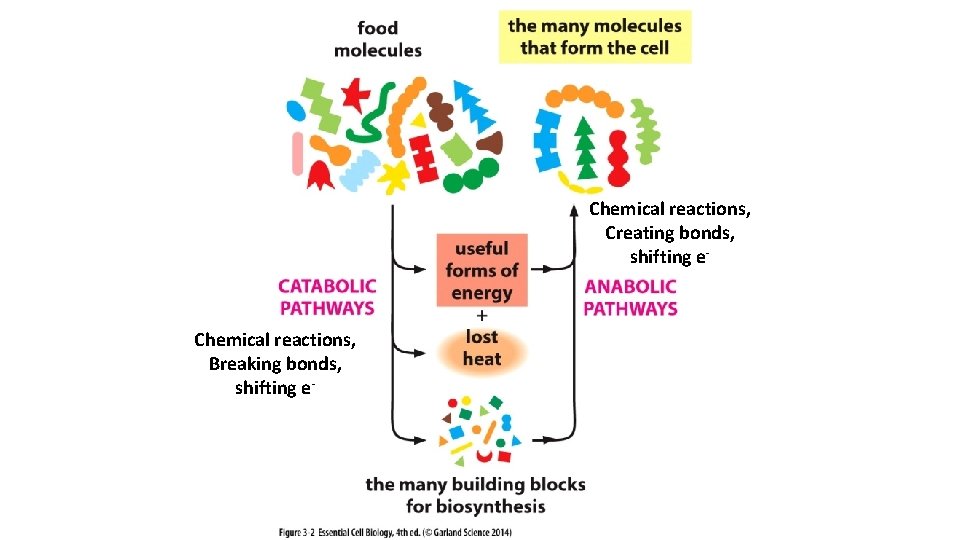
Chemical reactions, Creating bonds, shifting e- Chemical reactions, Breaking bonds, shifting e-

Moving electrons

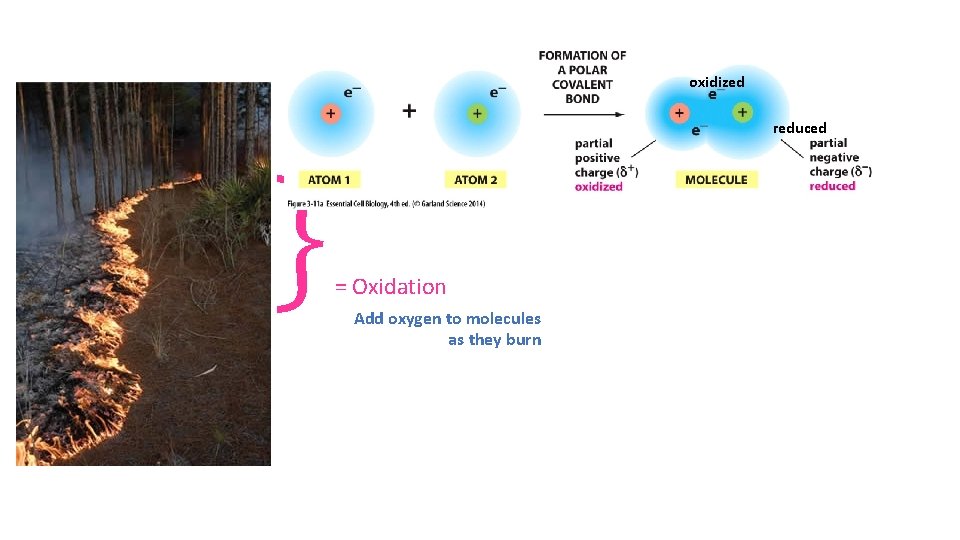
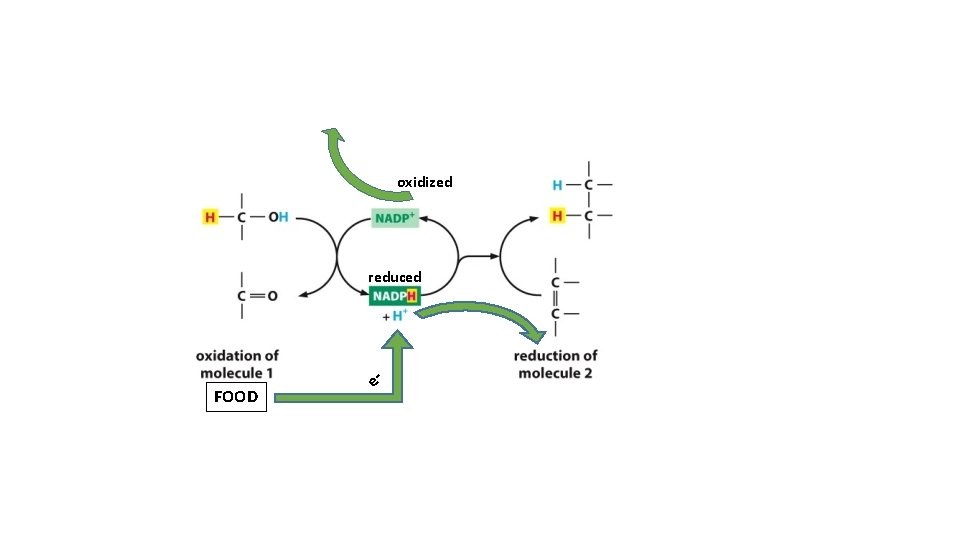
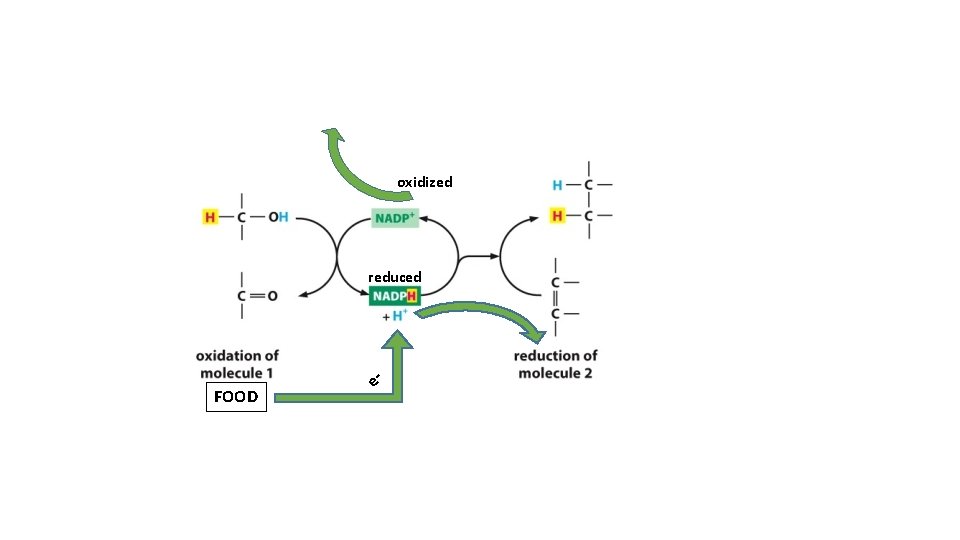
} oxidized reduced = Oxidation Add oxygen to molecules as they burn

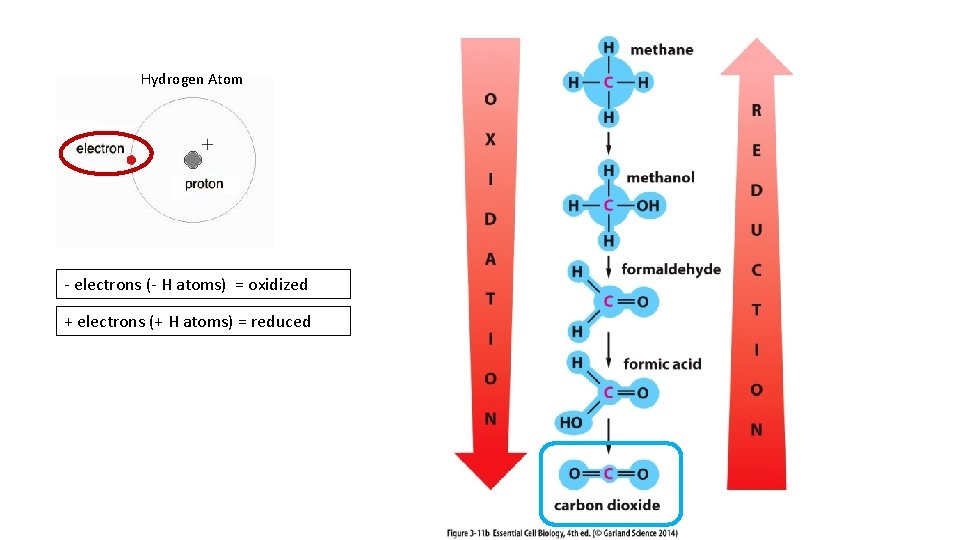
Hydrogen Atom - electrons (- H atoms) = oxidized + electrons (+ H atoms) = reduced

Usable (stored) Energy



oxidized reduced FOOD e-

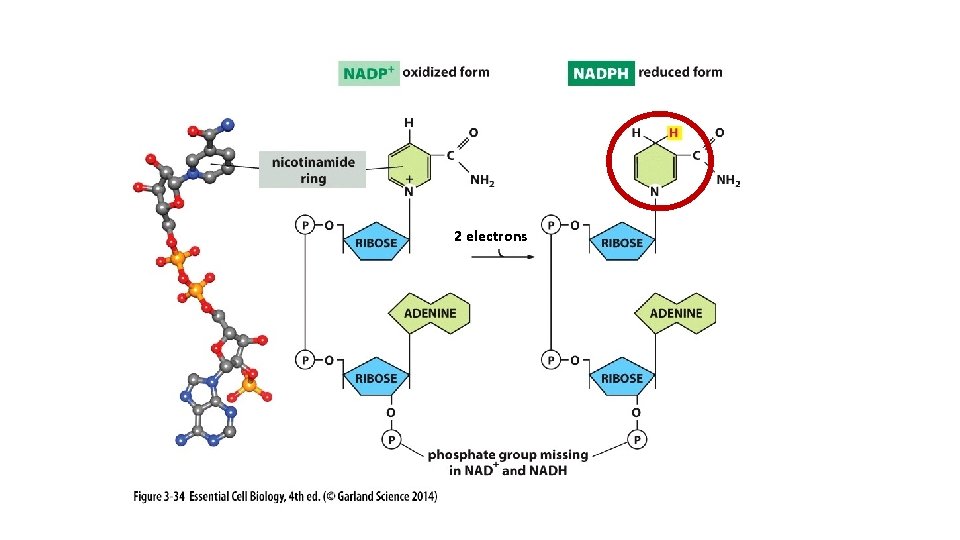
2 electrons

oxidized reduced FOOD e-

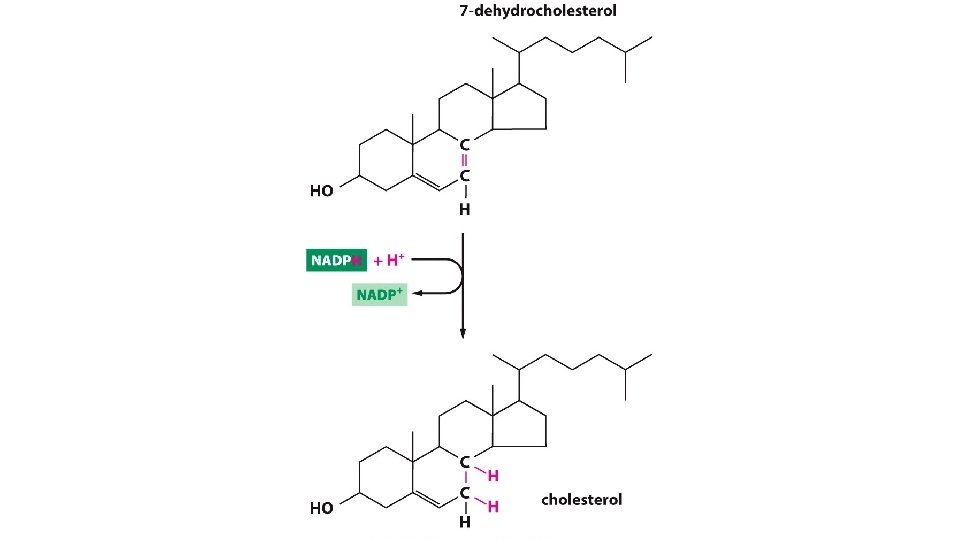
![Cytoplasmic NADPH NADP Cytoplasmic NADH NAD • Cytoplasmic [NADPH] > > [NADP+] • Cytoplasmic [NADH] < < [NAD+]](https://slidetodoc.com/presentation_image_h/0ec840fdb4703dbd124edebeabc4a555/image-18.jpg)
• Cytoplasmic [NADPH] > > [NADP+] • Cytoplasmic [NADH] < < [NAD+]

NAD+ NADH


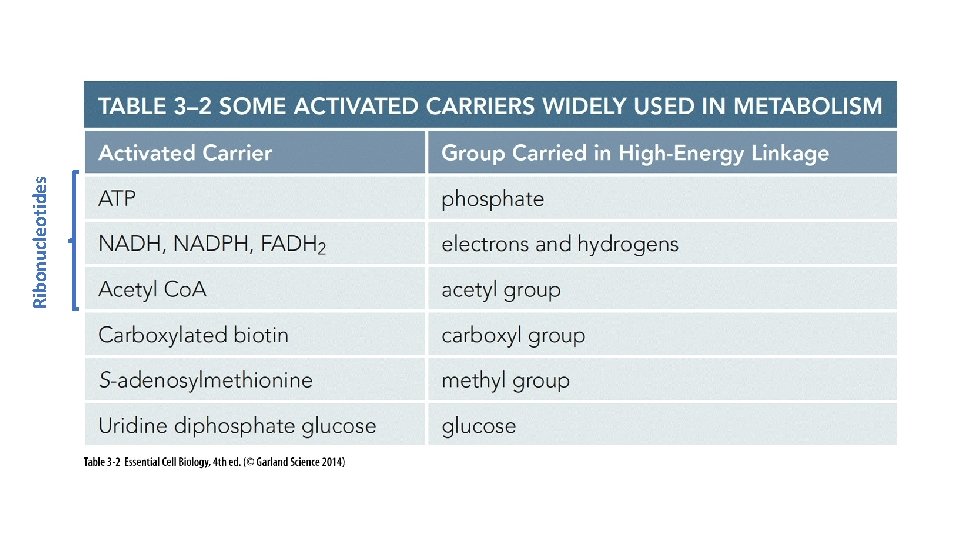
Ribonucleotides

When cells can use energy

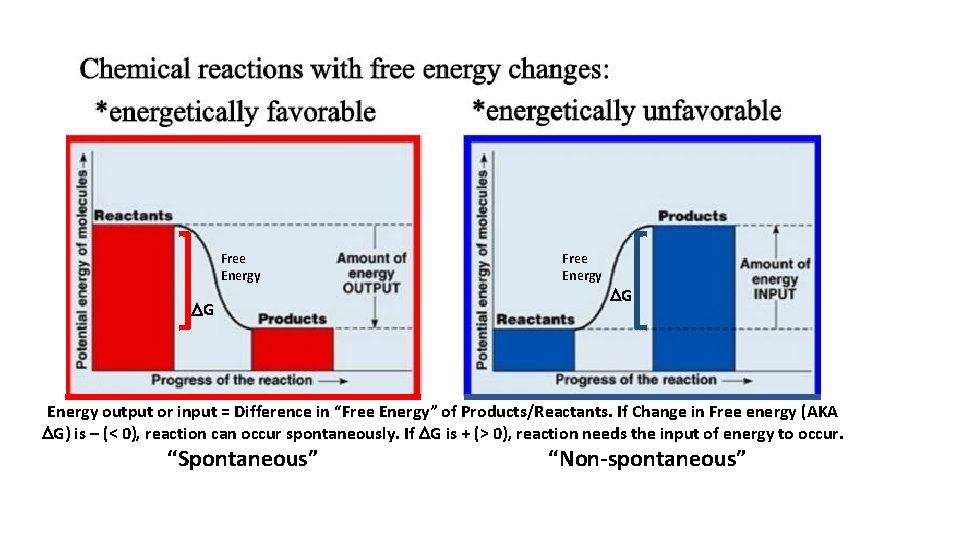
Free Energy DG Energy output or input = Difference in “Free Energy” of Products/Reactants. If Change in Free energy (AKA DG) is – (< 0), reaction can occur spontaneously. If DG is + (> 0), reaction needs the input of energy to occur. “Spontaneous” “Non-spontaneous”

DG < 0 DG > 0

• DG helps us predict whether a reaction will occur spontaneously. • DG also tells us how much energy will be produced in a spontaneous reaction (or will be needed to drive a nonspontaneous reaction).
![Potential energy At standard conditions when reactant product DGo X X tells Potential energy At standard conditions, when [reactant] = [product], DGo = X; X tells](https://slidetodoc.com/presentation_image_h/0ec840fdb4703dbd124edebeabc4a555/image-26.jpg)
Potential energy At standard conditions, when [reactant] = [product], DGo = X; X tells us how favorable (how much energy will be produced) a reaction is. Reaction progress
![DG 0 Without intervention as Y X reaction continues Y decreases and DG < 0 • Without intervention, as Y X reaction continues, [Y] decreases and](https://slidetodoc.com/presentation_image_h/0ec840fdb4703dbd124edebeabc4a555/image-27.jpg)
DG < 0 • Without intervention, as Y X reaction continues, [Y] decreases and [X] increases. • Therefore, DG changes (becomes more +) as the reaction proceeds. • Thus, reactions become less favorable as they proceed. • When a reaction reaches equilibrium, DG = 0. DG > 0



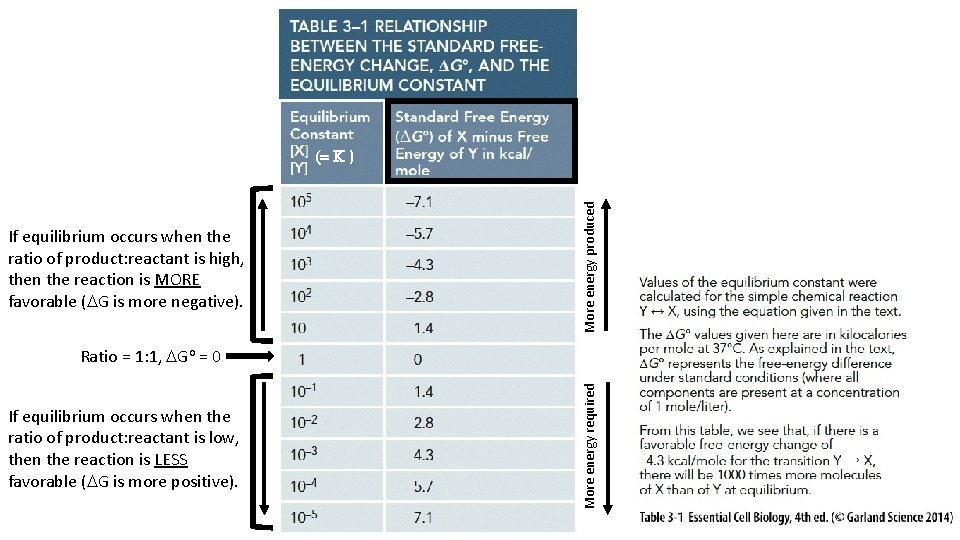
If equilibrium occurs when the ratio of product: reactant is high, then the reaction is MORE favorable (DG is more negative). More energy produced (= K ) If equilibrium occurs when the ratio of product: reactant is low, then the reaction is LESS favorable (DG is more positive). More energy required Ratio = 1: 1, DGo = 0
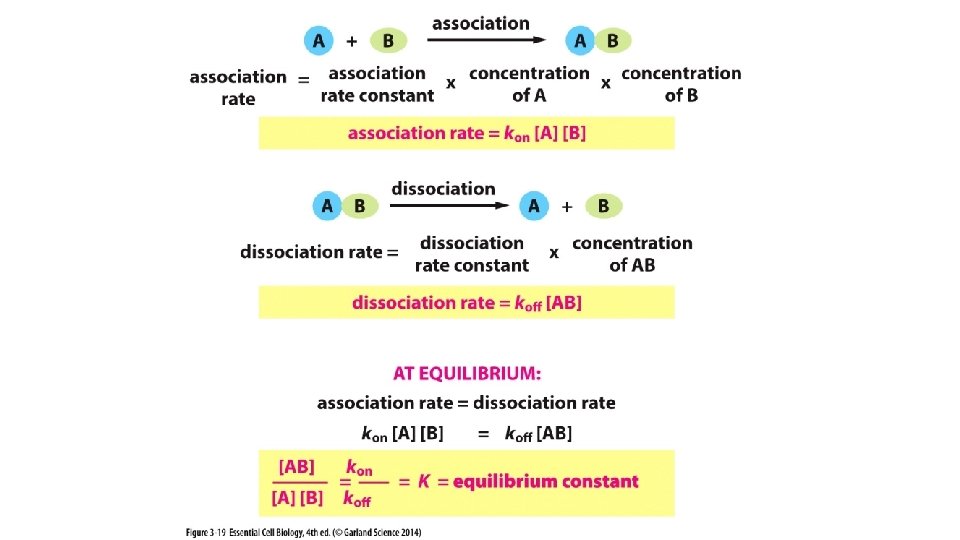
![Product Reactant when at equilibrium the Equilibrium Constant K [Product] [Reactant] when at equilibrium = the Equilibrium Constant (K)](https://slidetodoc.com/presentation_image_h/0ec840fdb4703dbd124edebeabc4a555/image-31.jpg)
[Product] [Reactant] when at equilibrium = the Equilibrium Constant (K)



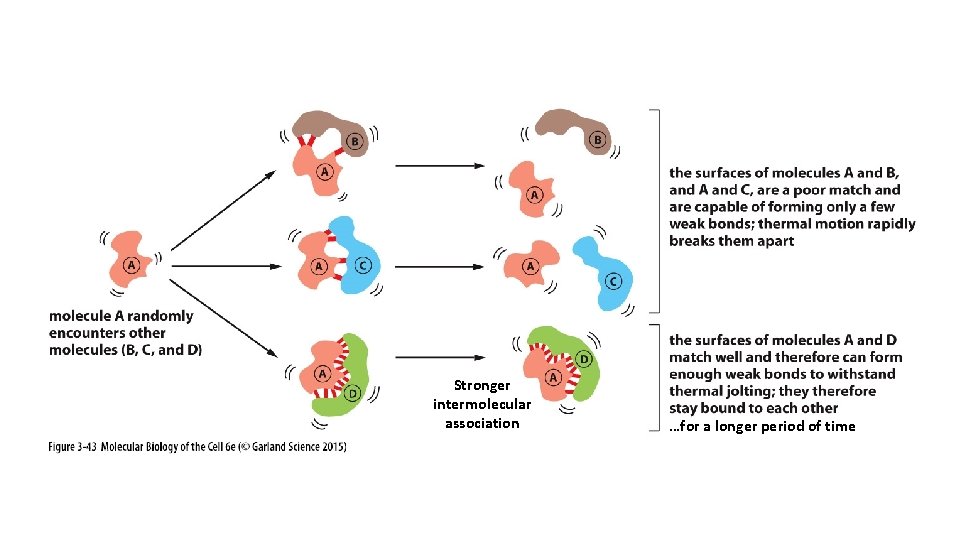
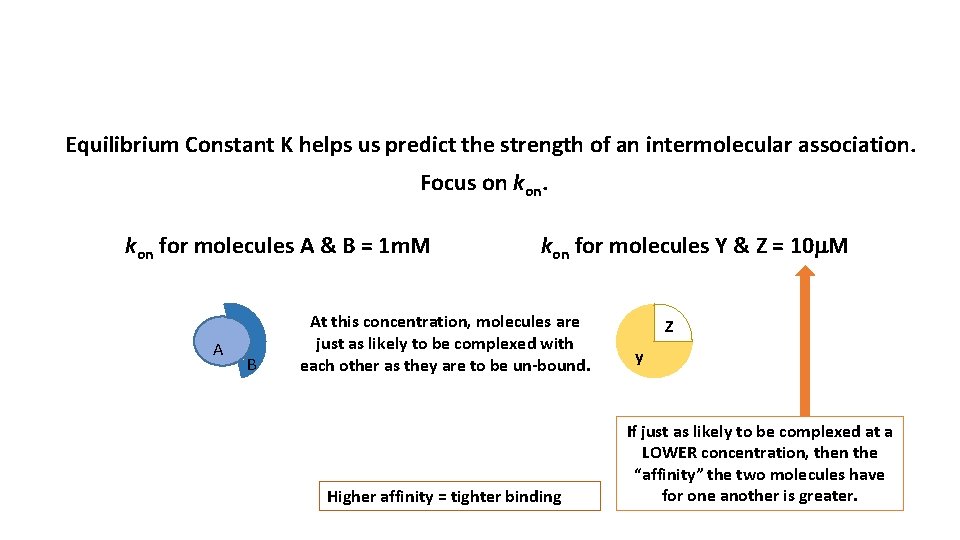
Equilibrium Constant K helps us predict the strength of an intermolecular association.

Stronger intermolecular association …for a longer period of time


Equilibrium Constant K helps us predict the strength of an intermolecular association. Focus on kon for molecules A & B = 1 m. M A B kon for molecules Y & Z = 10 m. M At this concentration, molecules are just as likely to be complexed with each other as they are to be un-bound. Higher affinity = tighter binding Z y If just as likely to be complexed at a LOWER concentration, then the “affinity” the two molecules have for one another is greater.

Finally, not all energetically favorable, “spontaneous” reactions are actually spontaneous.






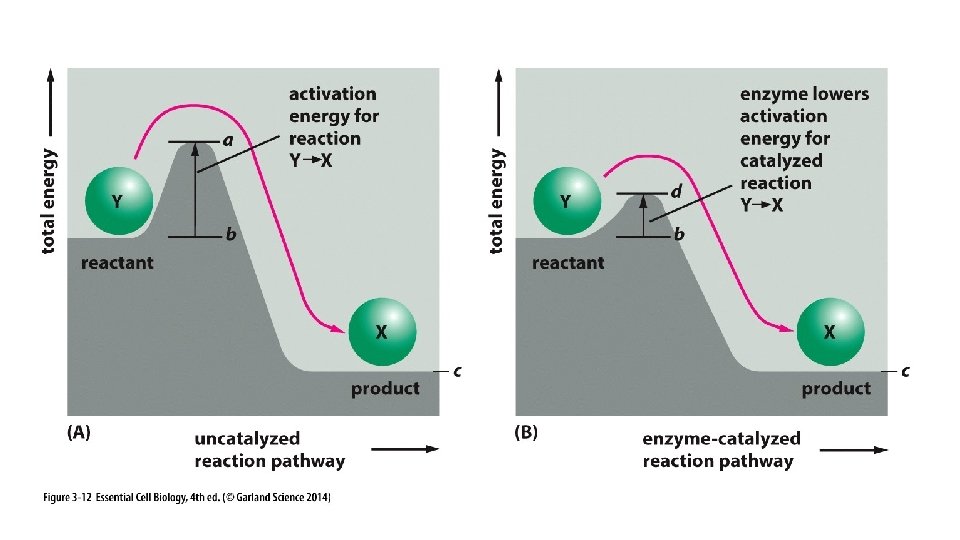
“Take Home” messages: • DG (free energy) is used to predict whether a reaction will occur spontaneously. • DG tells us how much energy will be produced if a reaction is spontaneous and if not, how much energy will be required to drive it. • DG is related to K, the equilibrium constant, which helps us predict the affinity between reactants. • Spontaneous reactions often must overcome an “activation energy”; this is facilitated by enzymes.
 Procedured
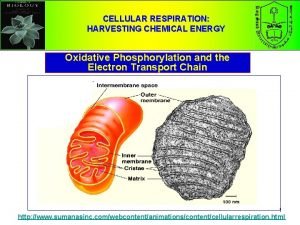
Procedured Cellular respiration harvesting chemical energy
Cellular respiration harvesting chemical energy Chapter 9 cellular respiration harvesting chemical energy
Chapter 9 cellular respiration harvesting chemical energy Chapter 9: cellular respiration: harvesting chemical energy
Chapter 9: cellular respiration: harvesting chemical energy Cellular respiration harvesting chemical energy
Cellular respiration harvesting chemical energy Cellular energy section 1 how organisms obtain energy
Cellular energy section 1 how organisms obtain energy Factitious disorder vs somatic symptom disorder
Factitious disorder vs somatic symptom disorder Enthalpy vs entropy
Enthalpy vs entropy Enthalpy entropy free energy
Enthalpy entropy free energy Dg = dg + rtlnq
Dg = dg + rtlnq Enthalpy entropy free energy
Enthalpy entropy free energy Entropy ap chem
Entropy ap chem What plant need for photosynthesis
What plant need for photosynthesis Equation for cell respiration
Equation for cell respiration What is the chemical formula of cellular respiration
What is the chemical formula of cellular respiration Strain energy stored due to torsion
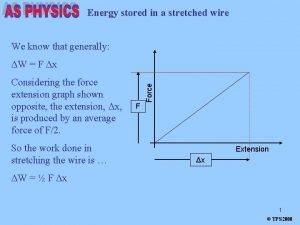
Strain energy stored due to torsion Energy stored in a spring
Energy stored in a spring Energy due to position
Energy due to position Stretched wire
Stretched wire Energy in a capacitor
Energy in a capacitor What type of energy is stored in atp
What type of energy is stored in atp The sudden release of energy stored in rocks causes a(n)
The sudden release of energy stored in rocks causes a(n) Stored energy solutions
Stored energy solutions Earth and beyond grade 7
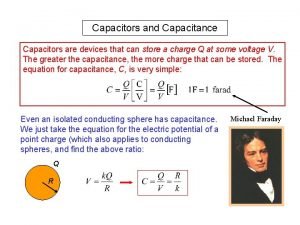
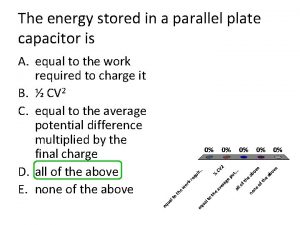
Earth and beyond grade 7 Energy stored in parallel plate capacitor
Energy stored in parallel plate capacitor Electrostatic energy density
Electrostatic energy density Energy stored in capacitors
Energy stored in capacitors Units of henry
Units of henry What is the nature of energy
What is the nature of energy Where is the energy stored in a parallel plate capacitor
Where is the energy stored in a parallel plate capacitor Energy stored in a capacitor
Energy stored in a capacitor Electric potential energy
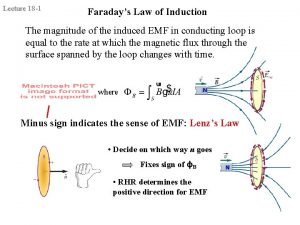
Electric potential energy Energy stored in inductor
Energy stored in inductor Energy of magnetic field formula
Energy of magnetic field formula Hand tool safety toolbox talk
Hand tool safety toolbox talk How is energy stored in a mousetrap
How is energy stored in a mousetrap As a roller coaster goes downhill
As a roller coaster goes downhill ________ converts light energy into chemical energy. *
________ converts light energy into chemical energy. * Energy example
Energy example Photosynthesis transforms light energy into chemical energy
Photosynthesis transforms light energy into chemical energy Db directive in 8051
Db directive in 8051 What are the types of advance directives
What are the types of advance directives Sic assembler directives
Sic assembler directives 8086 assembler directives
8086 assembler directives Advantages and disadvantages of advance directives
Advantages and disadvantages of advance directives