Cells and Batteries Electrical Cells and Batteries An
Cells and Batteries

Electrical Cells and Batteries • An Electrochemical cell is a device that converts chemical energy into electrical energy. • A battery is a collection of cells
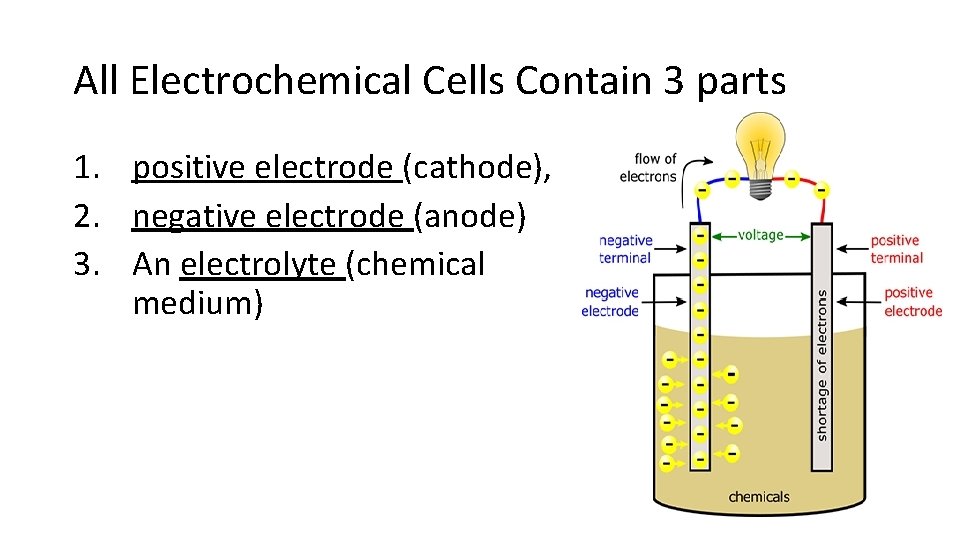
All Electrochemical Cells Contain 3 parts 1. positive electrode (cathode), 2. negative electrode (anode) 3. An electrolyte (chemical medium)
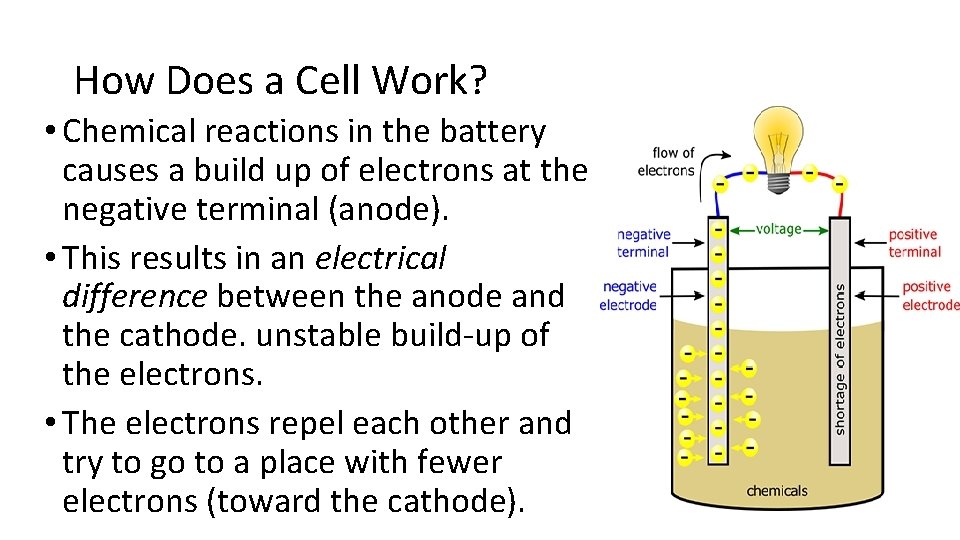
How Does a Cell Work? • Chemical reactions in the battery causes a build up of electrons at the negative terminal (anode). • This results in an electrical difference between the anode and the cathode. unstable build-up of the electrons. • The electrons repel each other and try to go to a place with fewer electrons (toward the cathode).
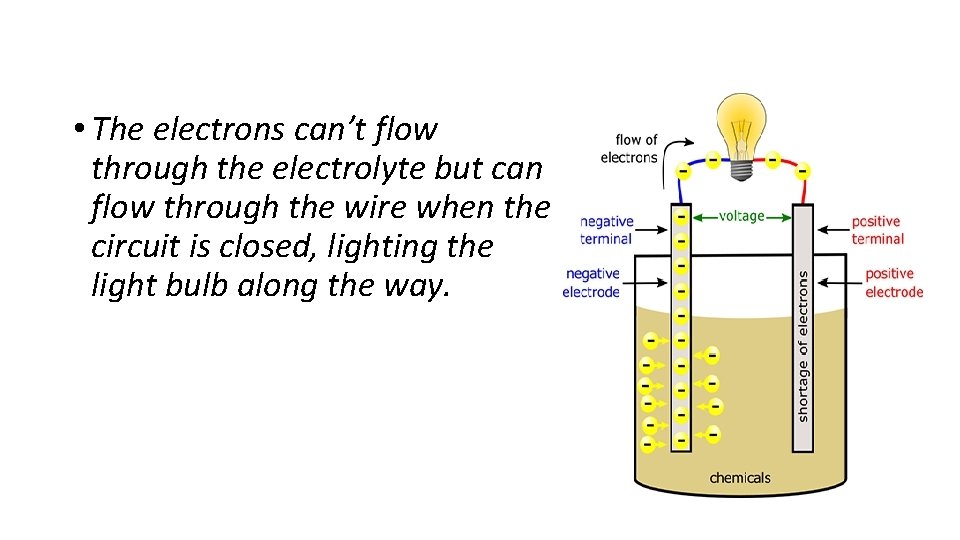
• The electrons can’t flow through the electrolyte but can flow through the wire when the circuit is closed, lighting the light bulb along the way.
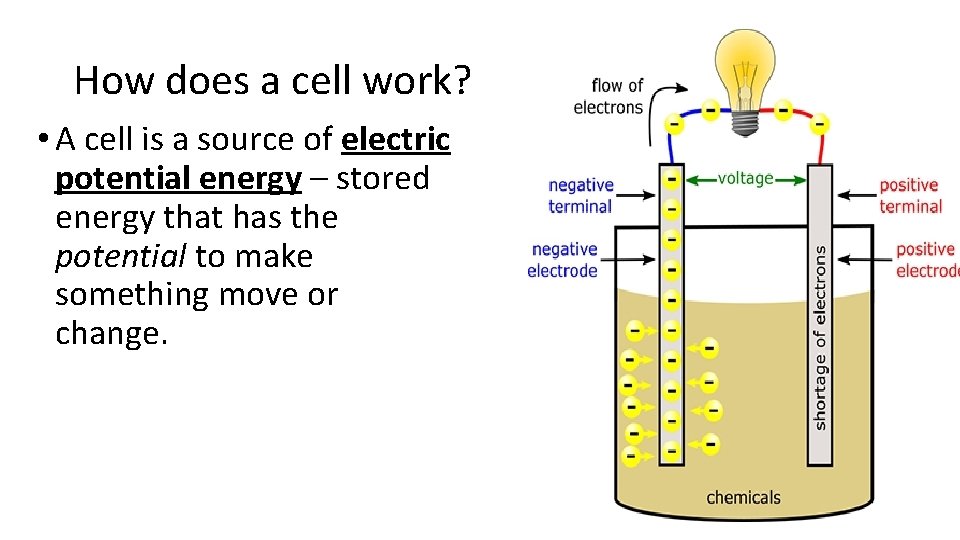
How does a cell work? • A cell is a source of electric potential energy – stored energy that has the potential to make something move or change.
What happens when you recharge a cell? • When you recharge a battery, you reverse the direction of the flow of electrons using another power source, such as solar panels. • The electrochemical processes happen in reverse, and the anode and cathode are restored to their original state and can again provide full power.

How batteries work • Ted Ed video https: //www. youtube. com/watch? v=9 OVtk 6 G 2 Tn. Q

Two types of Cells: • Primary (Dry Cell) • Secondary (Wet Cell)

Primary Cell (Dry Cell) • Dry cell contains a moist paste as an electrolyte. • Primary cell cannot be recharged because chemical reaction is irreversible.
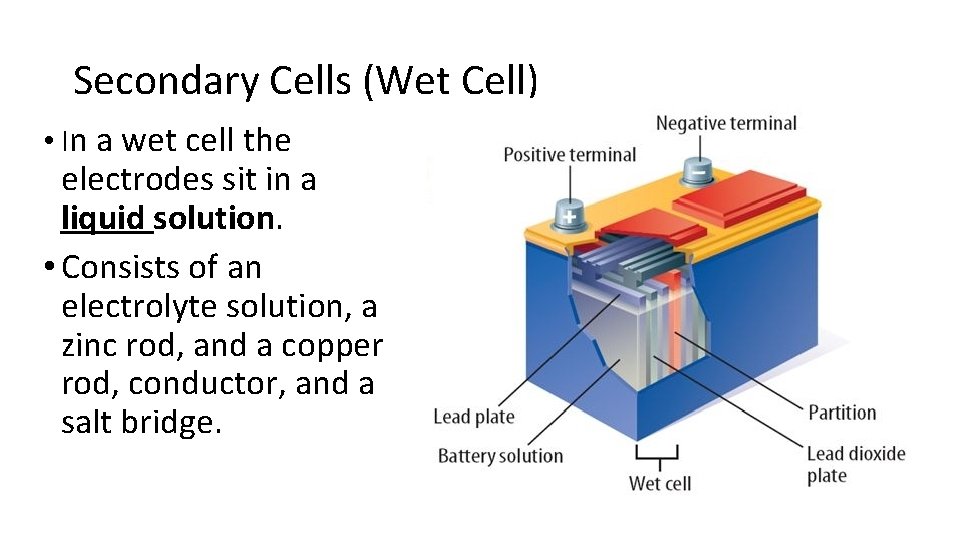
Secondary Cells (Wet Cell) • In a wet cell the electrodes sit in a liquid solution. • Consists of an electrolyte solution, a zinc rod, and a copper rod, conductor, and a salt bridge.

Secondary Cells (Wet Cell) • Secondary cell can be recharged using electrical energy to reverse the chemical reaction • Eg. Lead storage batteries (car battery) and • Eg. nickel – cadmium storage cell

Lemon Battery Explained: Sci Show • https: //www. youtube. com/watch? v=Ghbuh. T 1 GDp. I

How to make Battery from spare change • https: //www. youtube. com/watch? v=v. IHf. UJu 3 a. Ko • Lemon Battery Sci Show • https: //www. youtube. com/watch? v=Ghbuh. T 1 GDp. I

Practice • Make your own cell using pennies • CYU p 305 # 8 – 12 • Battery label WS
- Slides: 15