CellMatrix Interactions Dr Jeff Miner 7717 Wohl Clinic



- Slides: 41

Cell-Matrix Interactions Dr. Jeff Miner 7717 Wohl Clinic 362 -8235 minerj@wustl. edu

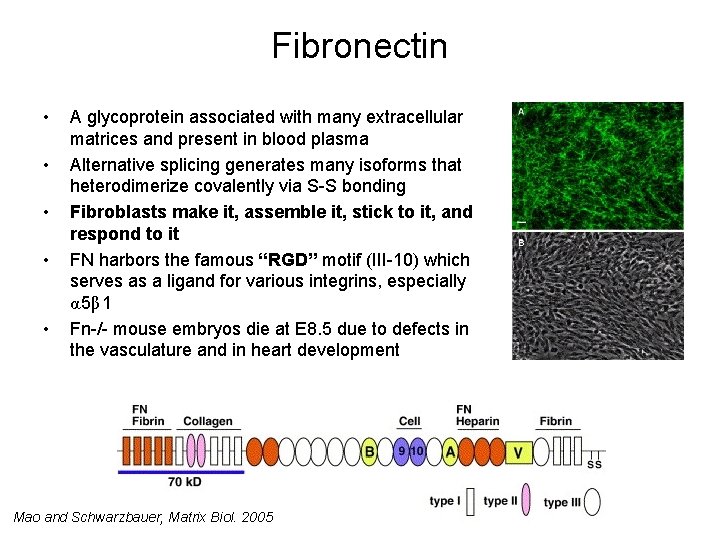
Fibronectin • • • A glycoprotein associated with many extracellular matrices and present in blood plasma Alternative splicing generates many isoforms that heterodimerize covalently via S-S bonding Fibroblasts make it, assemble it, stick to it, and respond to it FN harbors the famous “RGD” motif (III-10) which serves as a ligand for various integrins, especially α 5β 1 Fn-/- mouse embryos die at E 8. 5 due to defects in the vasculature and in heart development Mao and Schwarzbauer, Matrix Biol. 2005

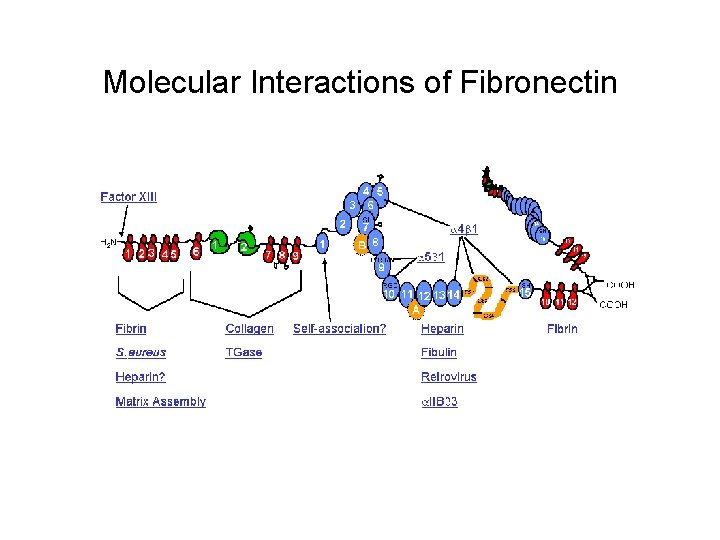
Molecular Interactions of Fibronectin

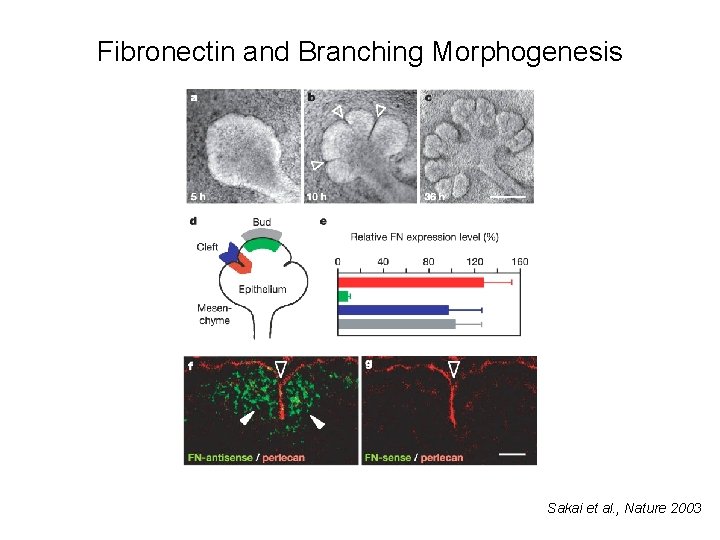
Fibronectin and Branching Morphogenesis Sakai et al. , Nature 2003

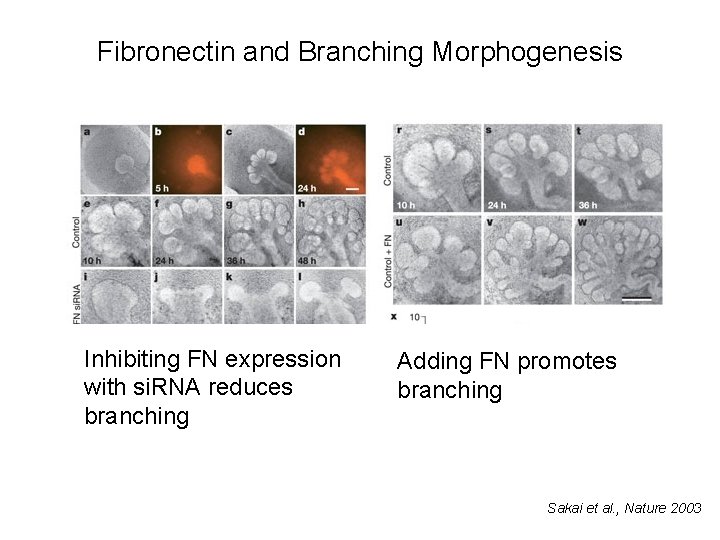
Fibronectin and Branching Morphogenesis Inhibiting FN expression with si. RNA reduces branching Adding FN promotes branching Sakai et al. , Nature 2003

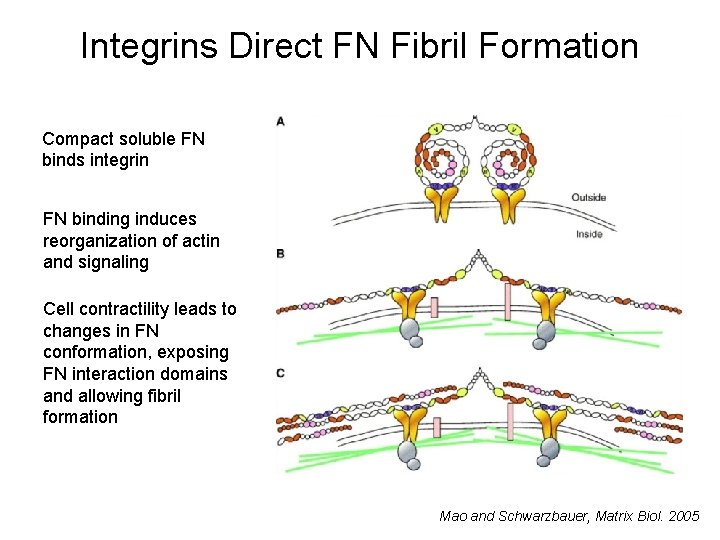
Integrins Direct FN Fibril Formation Compact soluble FN binds integrin FN binding induces reorganization of actin and signaling Cell contractility leads to changes in FN conformation, exposing FN interaction domains and allowing fibril formation Mao and Schwarzbauer, Matrix Biol. 2005

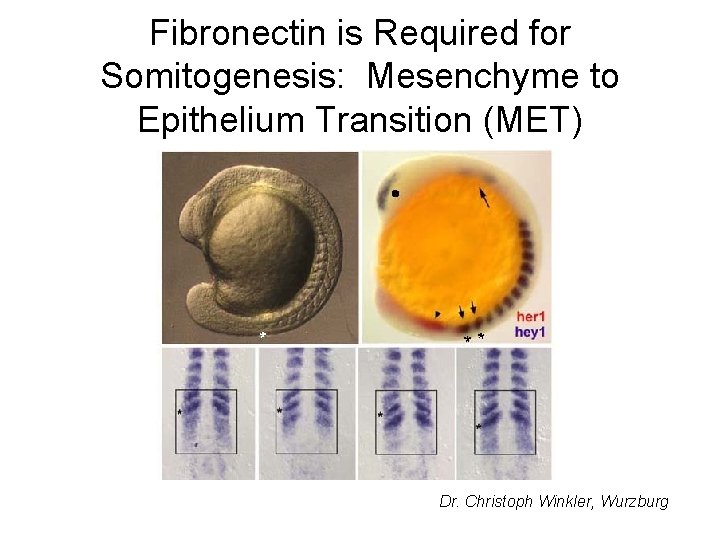
Fibronectin is Required for Somitogenesis: Mesenchyme to Epithelium Transition (MET) Dr. Christoph Winkler, Wurzburg

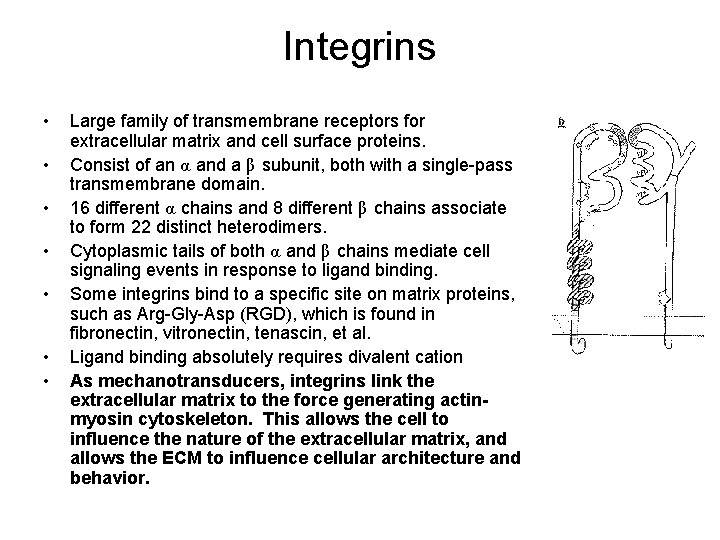
Integrins • • Large family of transmembrane receptors for extracellular matrix and cell surface proteins. Consist of an α and a β subunit, both with a single-pass transmembrane domain. 16 different α chains and 8 different β chains associate to form 22 distinct heterodimers. Cytoplasmic tails of both α and β chains mediate cell signaling events in response to ligand binding. Some integrins bind to a specific site on matrix proteins, such as Arg-Gly-Asp (RGD), which is found in fibronectin, vitronectin, tenascin, et al. Ligand binding absolutely requires divalent cation As mechanotransducers, integrins link the extracellular matrix to the force generating actinmyosin cytoskeleton. This allows the cell to influence the nature of the extracellular matrix, and allows the ECM to influence cellular architecture and behavior.

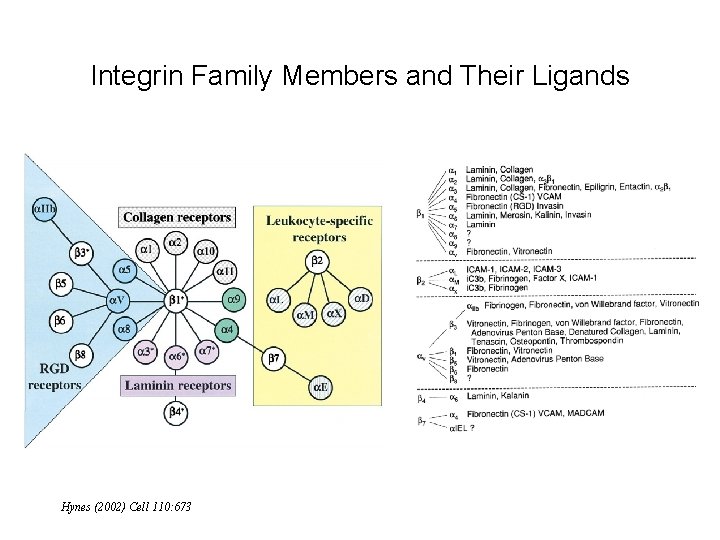
Integrin Family Members and Their Ligands Hynes (2002) Cell 110: 673

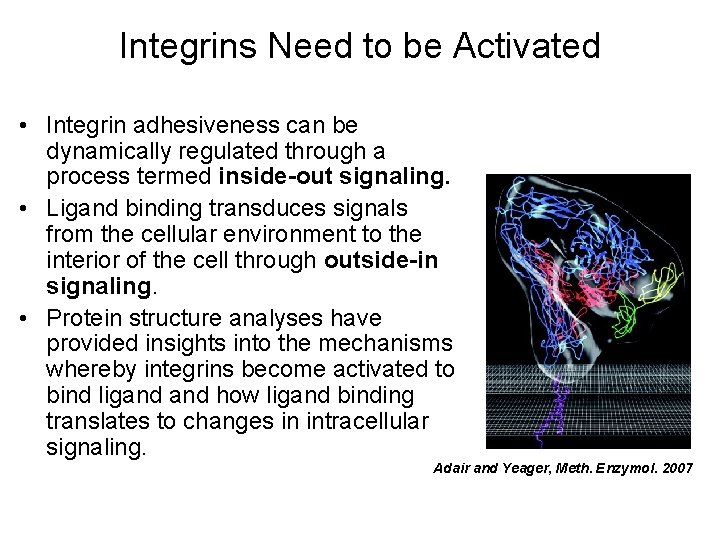
Integrins Need to be Activated • Integrin adhesiveness can be dynamically regulated through a process termed inside-out signaling. • Ligand binding transduces signals from the cellular environment to the interior of the cell through outside-in signaling. • Protein structure analyses have provided insights into the mechanisms whereby integrins become activated to bind ligand how ligand binding translates to changes in intracellular signaling. Adair and Yeager, Meth. Enzymol. 2007

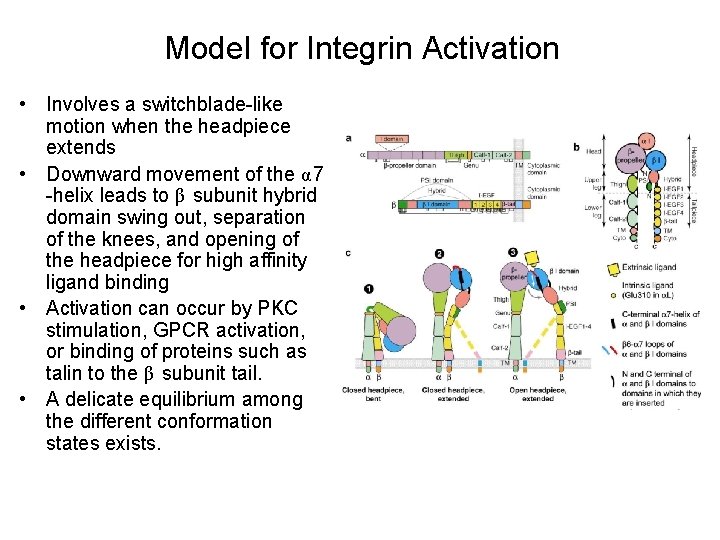
Model for Integrin Activation • Involves a switchblade-like motion when the headpiece extends • Downward movement of the α 7 -helix leads to β subunit hybrid domain swing out, separation of the knees, and opening of the headpiece for high affinity ligand binding • Activation can occur by PKC stimulation, GPCR activation, or binding of proteins such as talin to the β subunit tail. • A delicate equilibrium among the different conformation states exists.

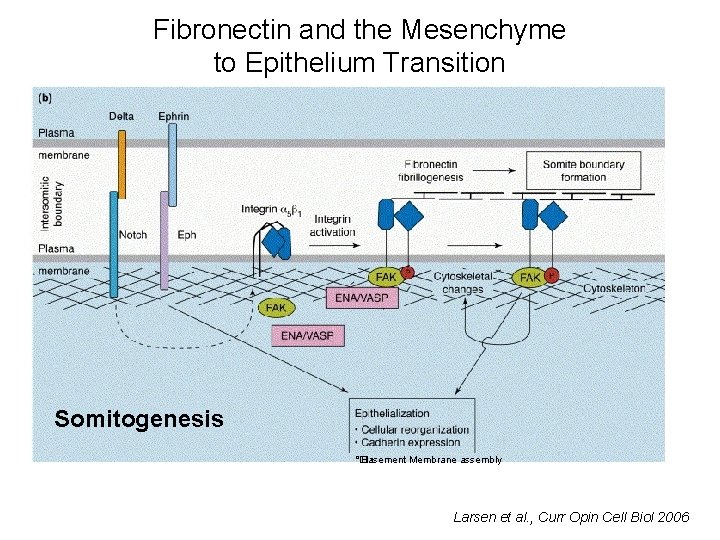
Fibronectin and the Mesenchyme to Epithelium Transition Somitogenesis °� • Basement Membrane assembly Larsen et al. , Curr Opin Cell Biol 2006

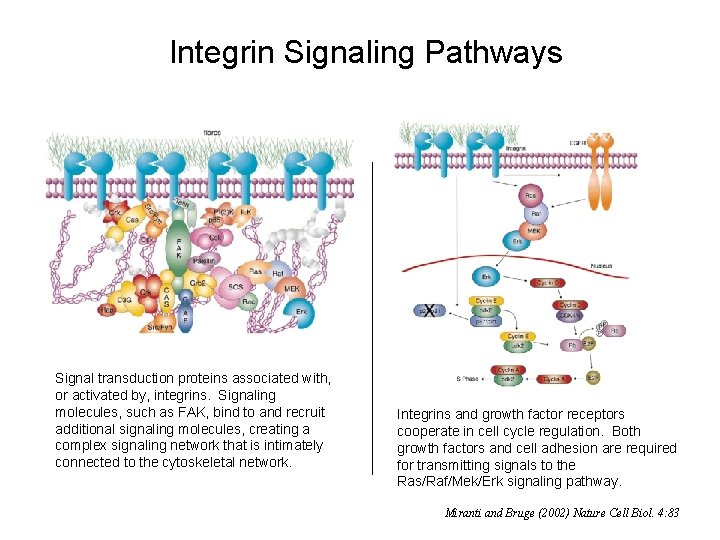
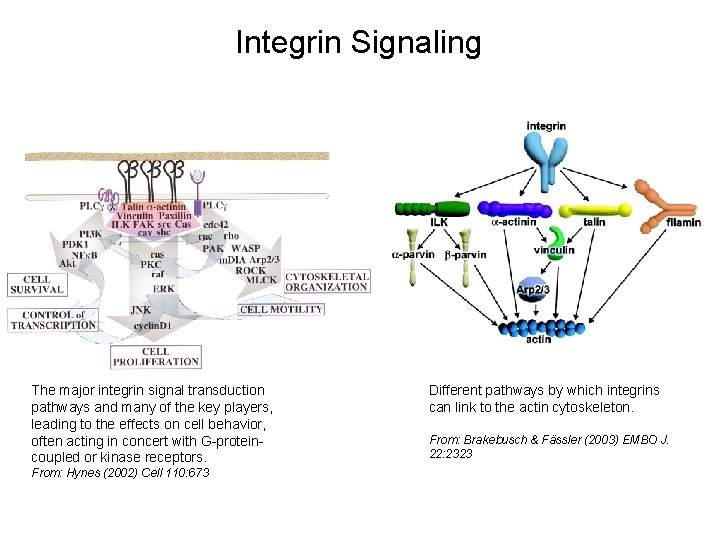
Integrin Signaling Pathways Signal transduction proteins associated with, or activated by, integrins. Signaling molecules, such as FAK, bind to and recruit additional signaling molecules, creating a complex signaling network that is intimately connected to the cytoskeletal network. Integrins and growth factor receptors cooperate in cell cycle regulation. Both growth factors and cell adhesion are required for transmitting signals to the Ras/Raf/Mek/Erk signaling pathway. Miranti and Bruge (2002) Nature Cell Biol. 4: 83

Integrin Signaling The major integrin signal transduction pathways and many of the key players, leading to the effects on cell behavior, often acting in concert with G-proteincoupled or kinase receptors. From: Hynes (2002) Cell 110: 673 Different pathways by which integrins can link to the actin cytoskeleton. From: Brakebusch & Fässler (2003) EMBO J. 22: 2323

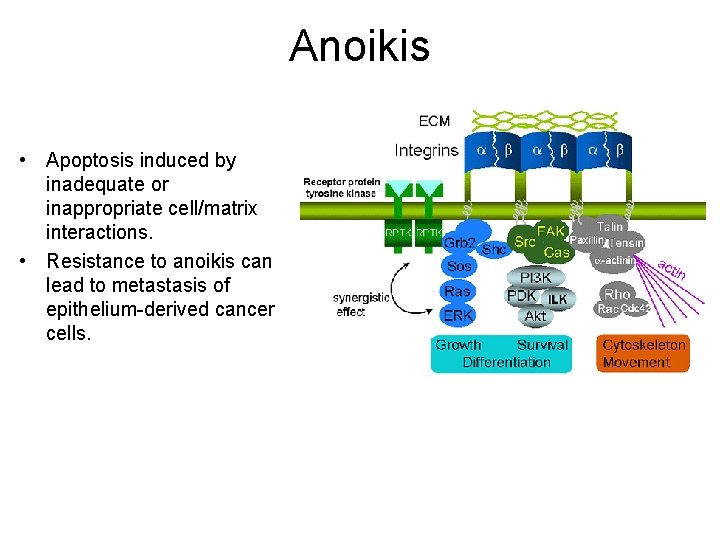
Anoikis • Apoptosis induced by inadequate or inappropriate cell/matrix interactions. • Resistance to anoikis can lead to metastasis of epithelium-derived cancer cells.

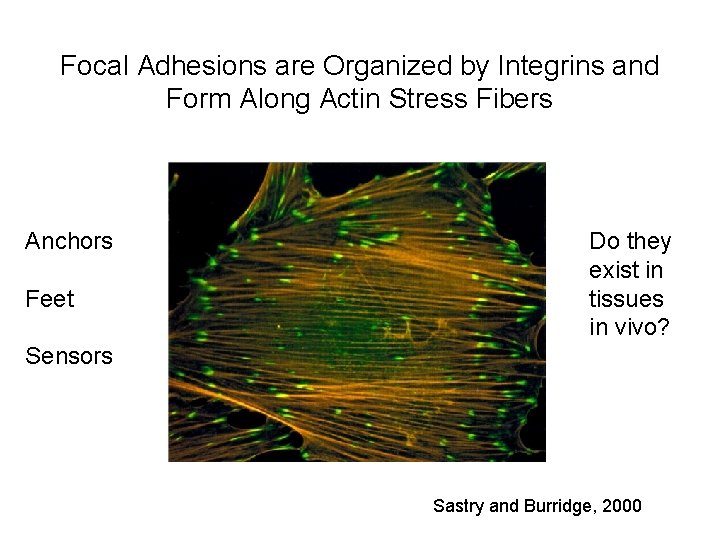
Focal Adhesions are Organized by Integrins and Form Along Actin Stress Fibers Anchors Feet Do they exist in tissues in vivo? Sensors Sastry and Burridge, 2000

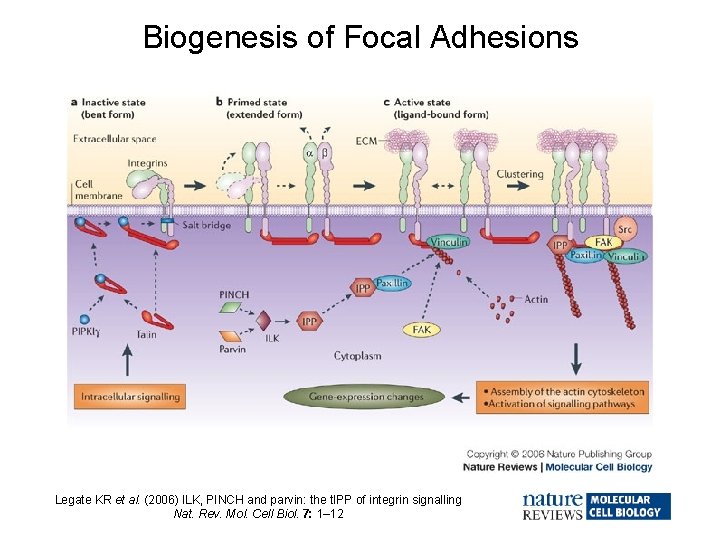
Biogenesis of Focal Adhesions Legate KR et al. (2006) ILK, PINCH and parvin: the t. IPP of integrin signalling Nat. Rev. Mol. Cell Biol. 7: 1– 12

Receptors for the Basement Membrane • Cells are thought to recognize the basement membrane through receptors that interact with specific basement membrane components, primarily with laminin. – – Integrins Dystroglycan Ig-superfamily transmembrane receptor (Lutheran/B-CAM) Syndecans--transmembrane receptors with HSPG side chains in their ectodomains. • Binding of receptors to the basement membrane can result in signal transduction and alterations in cell behavior

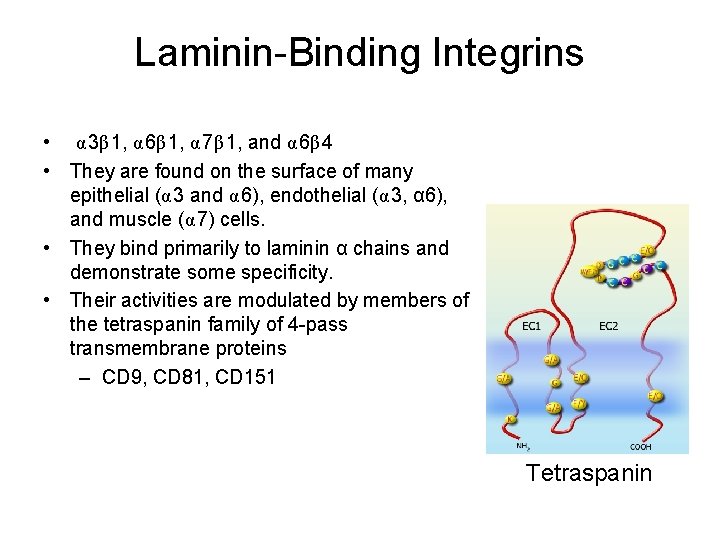
Laminin-Binding Integrins • α 3β 1, α 6β 1, α 7β 1, and α 6β 4 • They are found on the surface of many epithelial (α 3 and α 6), endothelial (α 3, α 6), and muscle (α 7) cells. • They bind primarily to laminin α chains and demonstrate some specificity. • Their activities are modulated by members of the tetraspanin family of 4 -pass transmembrane proteins – CD 9, CD 81, CD 151 Tetraspanin

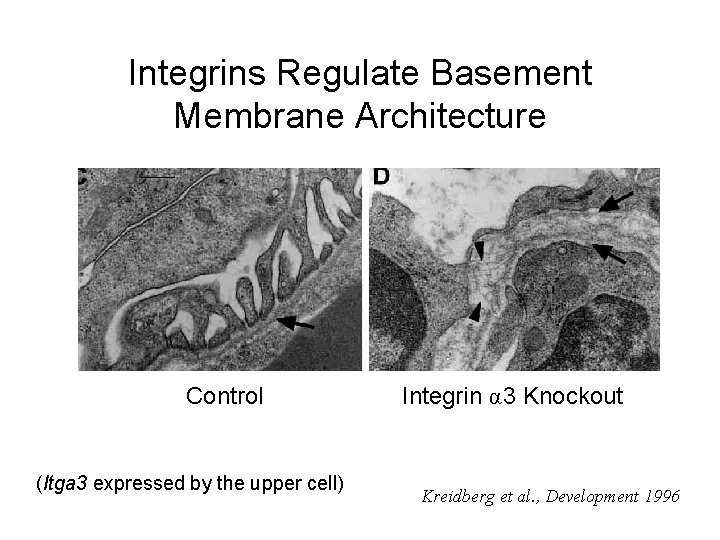
Integrins Regulate Basement Membrane Architecture Control (Itga 3 expressed by the upper cell) Integrin α 3 Knockout Kreidberg et al. , Development 1996

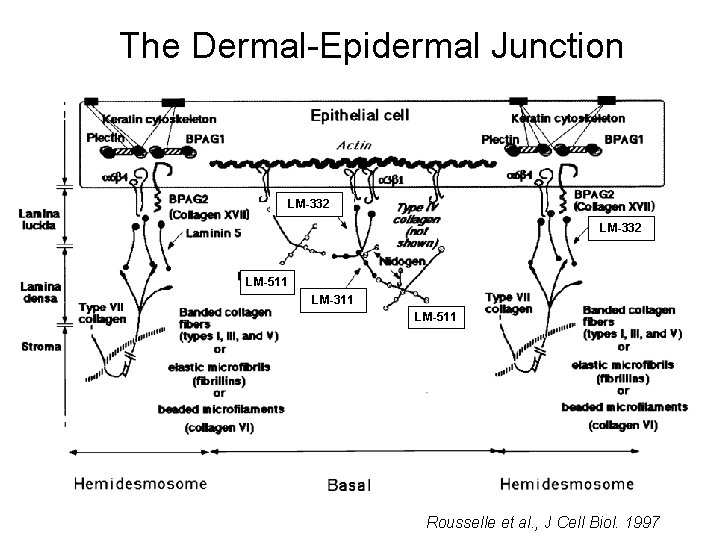
The Dermal-Epidermal Junction

The Dermal-Epidermal Junction LM-332 Laminin-332 LM-511 LM-311 LM-511 Rousselle et al. , J Cell Biol. 1997

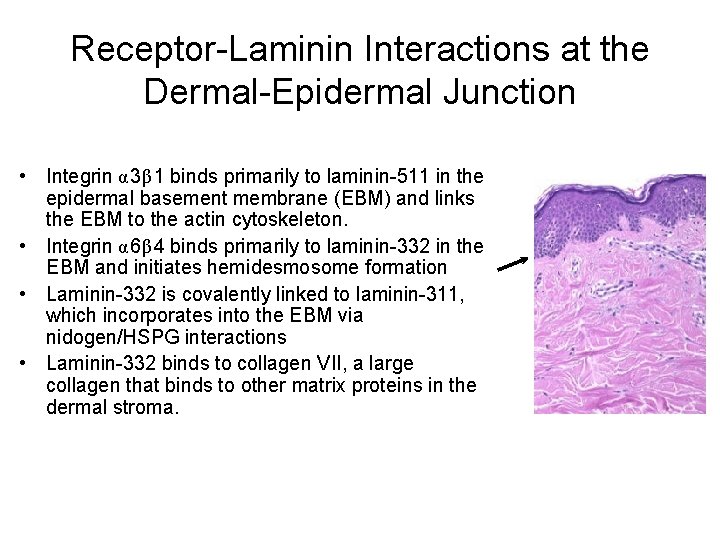
Receptor-Laminin Interactions at the Dermal-Epidermal Junction • Integrin α 3β 1 binds primarily to laminin-511 in the epidermal basement membrane (EBM) and links the EBM to the actin cytoskeleton. • Integrin α 6β 4 binds primarily to laminin-332 in the EBM and initiates hemidesmosome formation • Laminin-332 is covalently linked to laminin-311, which incorporates into the EBM via nidogen/HSPG interactions • Laminin-332 binds to collagen VII, a large collagen that binds to other matrix proteins in the dermal stroma.

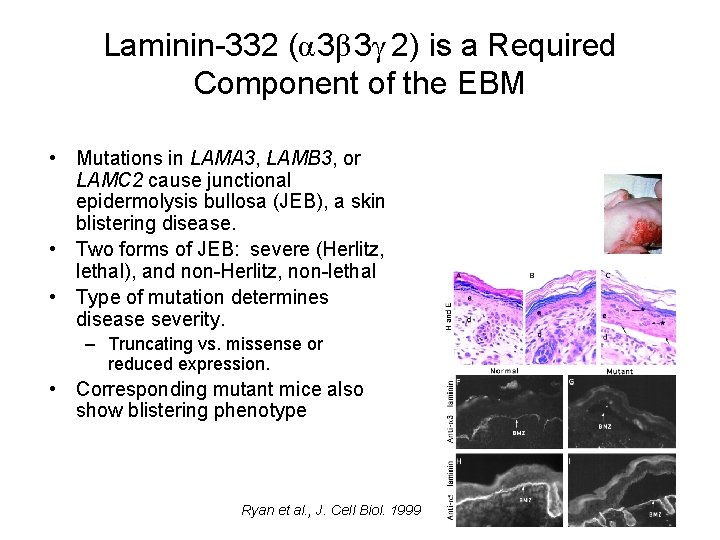
Laminin-332 (α 3β 3γ 2) is a Required Component of the EBM • Mutations in LAMA 3, LAMB 3, or LAMC 2 cause junctional epidermolysis bullosa (JEB), a skin blistering disease. • Two forms of JEB: severe (Herlitz, lethal), and non-Herlitz, non-lethal • Type of mutation determines disease severity. – Truncating vs. missense or reduced expression. • Corresponding mutant mice also show blistering phenotype Ryan et al. , J. Cell Biol. 1999

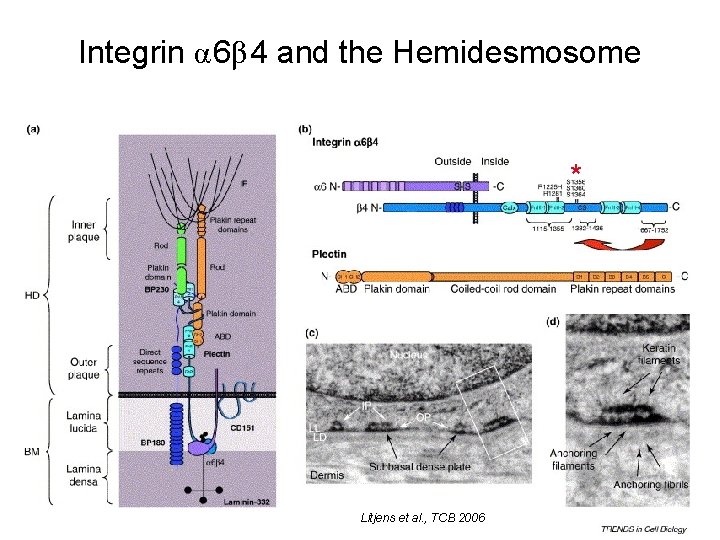
Integrin α 6β 4 and the Hemidesmosome * Litjens et al. , TCB 2006

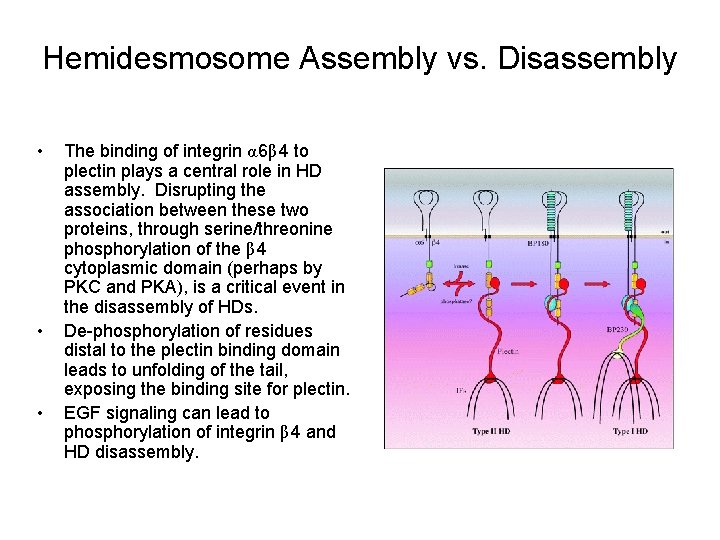
Hemidesmosome Assembly vs. Disassembly • • • The binding of integrin α 6β 4 to plectin plays a central role in HD assembly. Disrupting the association between these two proteins, through serine/threonine phosphorylation of the β 4 cytoplasmic domain (perhaps by PKC and PKA), is a critical event in the disassembly of HDs. De-phosphorylation of residues distal to the plectin binding domain leads to unfolding of the tail, exposing the binding site for plectin. EGF signaling can lead to phosphorylation of integrin β 4 and HD disassembly.

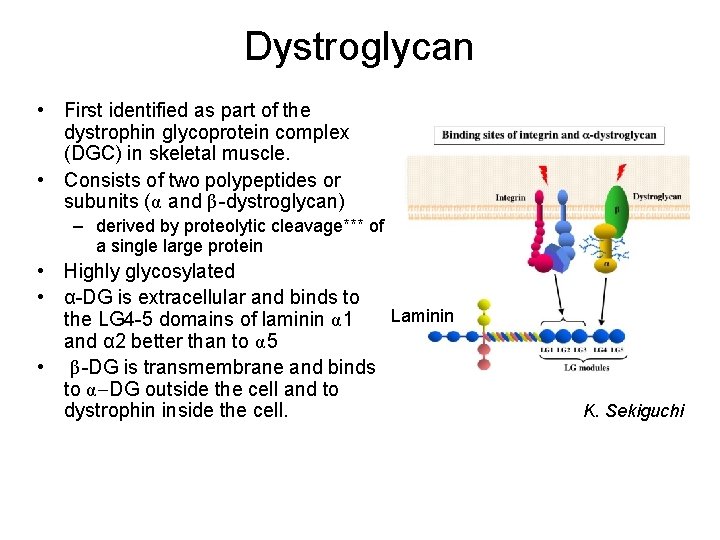
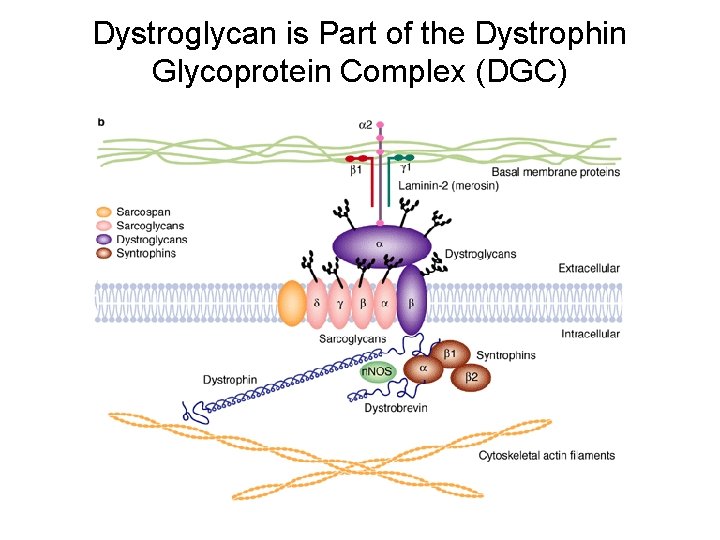
Dystroglycan • First identified as part of the dystrophin glycoprotein complex (DGC) in skeletal muscle. • Consists of two polypeptides or subunits (α and β-dystroglycan) – derived by proteolytic cleavage*** of a single large protein • Highly glycosylated • α-DG is extracellular and binds to Laminin the LG 4 -5 domains of laminin α 1 and α 2 better than to α 5 • β-DG is transmembrane and binds to α-DG outside the cell and to dystrophin inside the cell. K. Sekiguchi

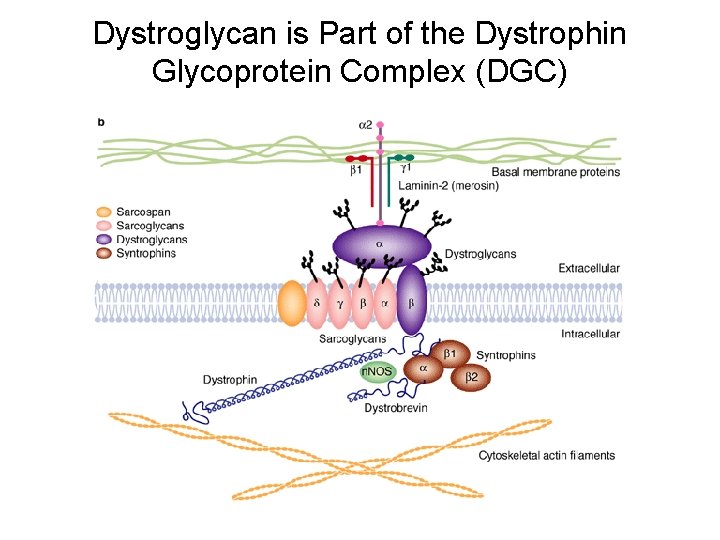
Dystroglycan is Part of the Dystrophin Glycoprotein Complex (DGC)

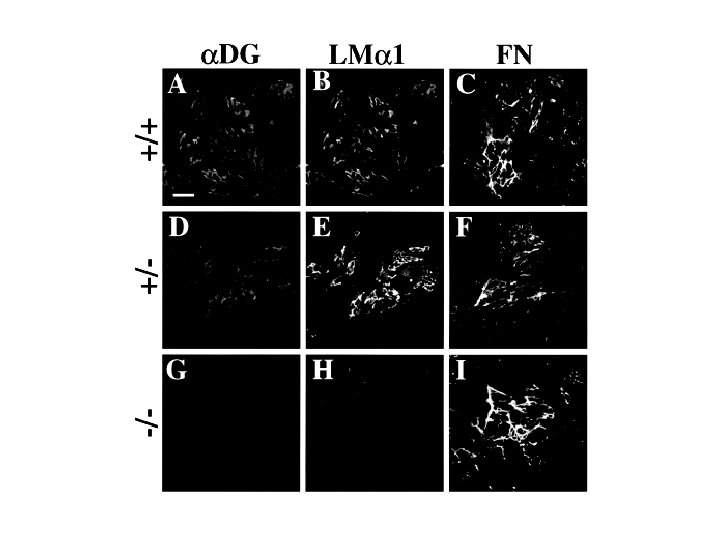
Dystroglycan • Some evidence suggests that dystroglycan is involved in and perhaps necessary for laminin polymerization at the surface of cells, which initiates basement membrane formation. • Dystroglycan KO embryonic stem cells cannot assemble soluble laminin at the cell surface.


Dystroglycan • Some evidence suggests that dystroglycan is involved in and perhaps necessary for laminin polymerization at the surface of cells, which initiates basement membrane formation. • Dystroglycan KO embryonic stem cells cannot assemble soluble laminin at their cell surfaces. • Dystroglycan KO has no Reichert’s basement membrane, dies at ~E 5 to E 6 (just after implantation into the uterus).

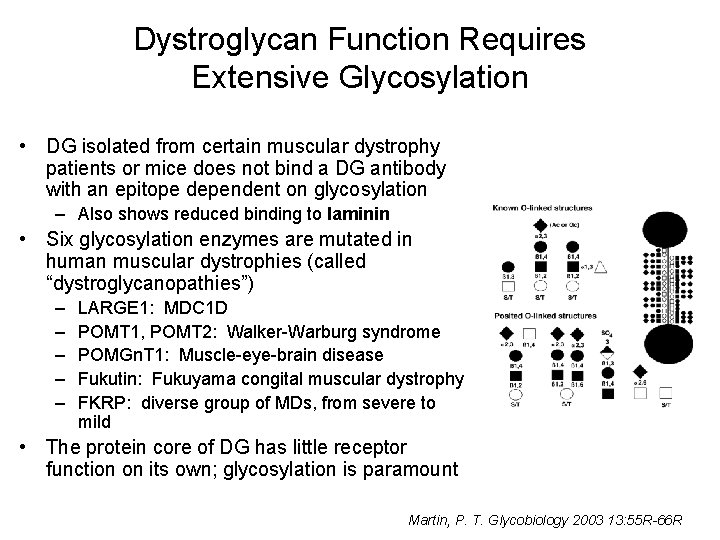
Dystroglycan Function Requires Extensive Glycosylation • DG isolated from certain muscular dystrophy patients or mice does not bind a DG antibody with an epitope dependent on glycosylation – Also shows reduced binding to laminin • Six glycosylation enzymes are mutated in human muscular dystrophies (called “dystroglycanopathies”) – – – LARGE 1: MDC 1 D POMT 1, POMT 2: Walker-Warburg syndrome POMGn. T 1: Muscle-eye-brain disease Fukutin: Fukuyama congital muscular dystrophy FKRP: diverse group of MDs, from severe to mild • The protein core of DG has little receptor function on its own; glycosylation is paramount Martin, P. T. Glycobiology 2003 13: 55 R-66 R

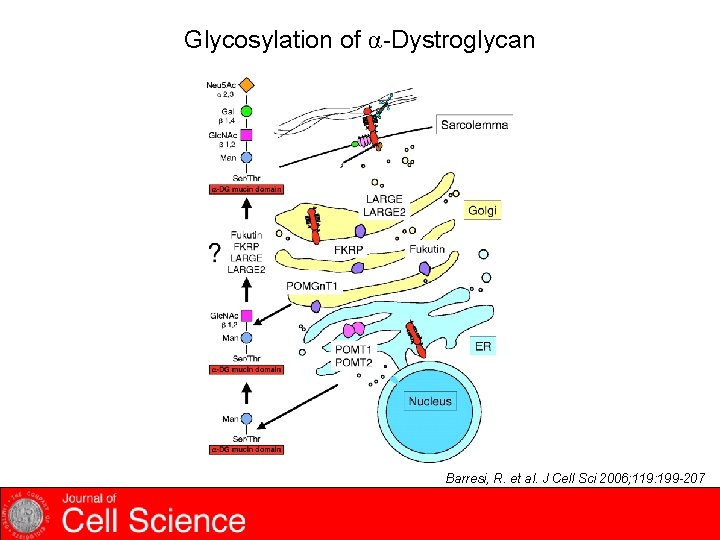
Glycosylation of α-Dystroglycan Barresi, R. et al. J Cell Sci 2006; 119: 199 -207

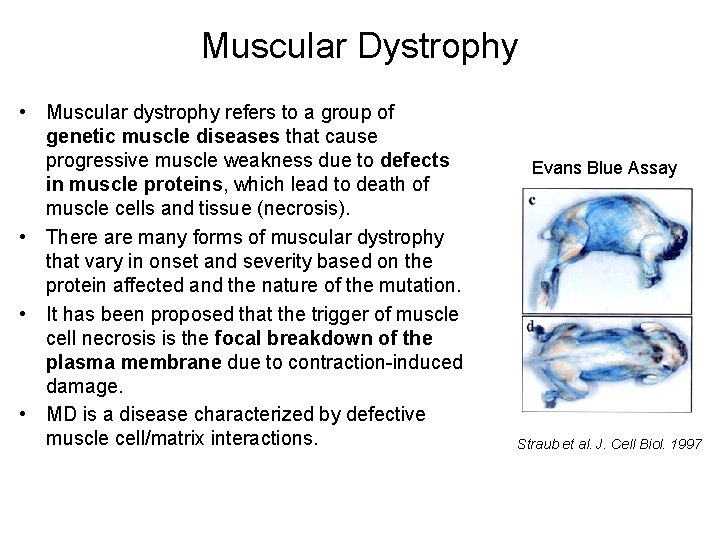
Muscular Dystrophy • Muscular dystrophy refers to a group of genetic muscle diseases that cause progressive muscle weakness due to defects in muscle proteins, which lead to death of muscle cells and tissue (necrosis). • There are many forms of muscular dystrophy that vary in onset and severity based on the protein affected and the nature of the mutation. • It has been proposed that the trigger of muscle cell necrosis is the focal breakdown of the plasma membrane due to contraction-induced damage. • MD is a disease characterized by defective muscle cell/matrix interactions. Evans Blue Assay Straub et al. J. Cell Biol. 1997

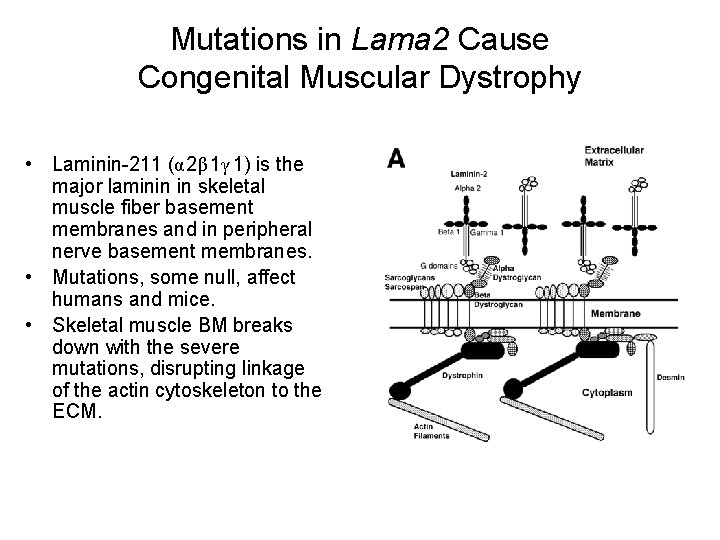
Mutations in Lama 2 Cause Congenital Muscular Dystrophy • Laminin-211 (α 2β 1γ 1) is the major laminin in skeletal muscle fiber basement membranes and in peripheral nerve basement membranes. • Mutations, some null, affect humans and mice. • Skeletal muscle BM breaks down with the severe mutations, disrupting linkage of the actin cytoskeleton to the ECM.

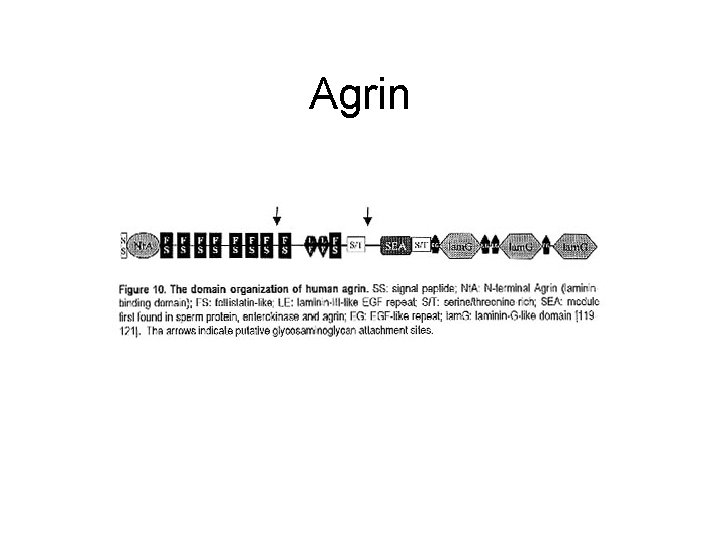
Agrin

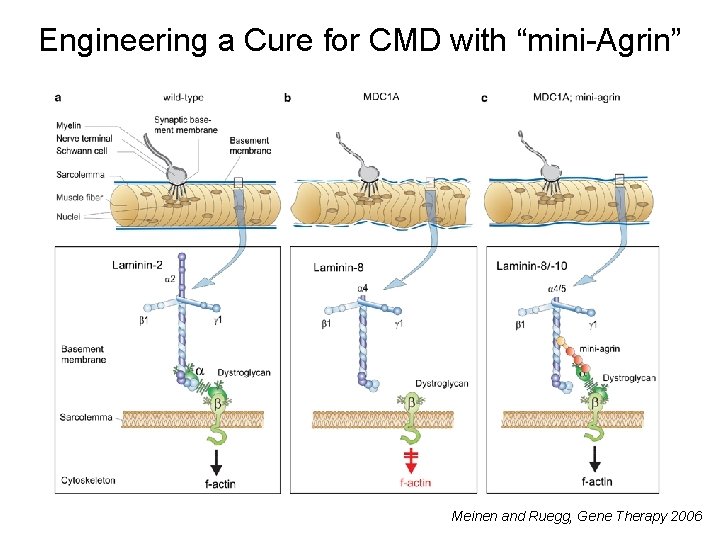
Engineering a Cure for CMD with “mini-Agrin” Meinen and Ruegg, Gene Therapy 2006

Dystroglycan is Part of the Dystrophin Glycoprotein Complex (DGC)

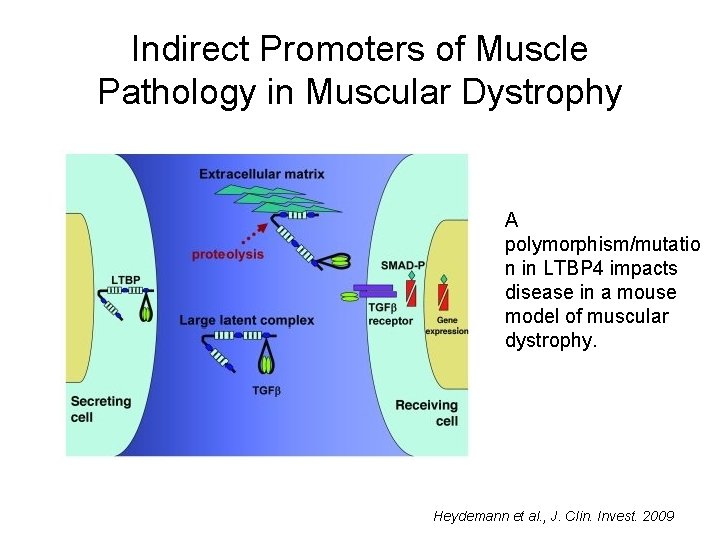
Indirect Promoters of Muscle Pathology in Muscular Dystrophy A polymorphism/mutatio n in LTBP 4 impacts disease in a mouse model of muscular dystrophy. Heydemann et al. , J. Clin. Invest. 2009

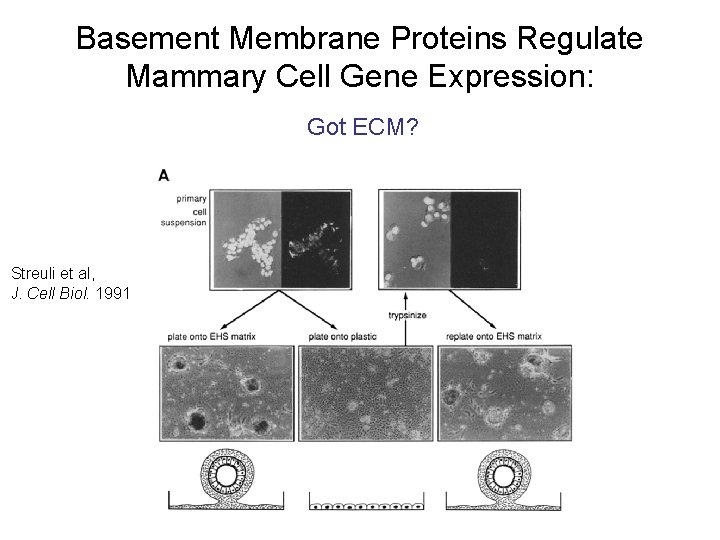
Basement Membrane Proteins Regulate Mammary Cell Gene Expression: Got ECM? Streuli et al, J. Cell Biol. 1991

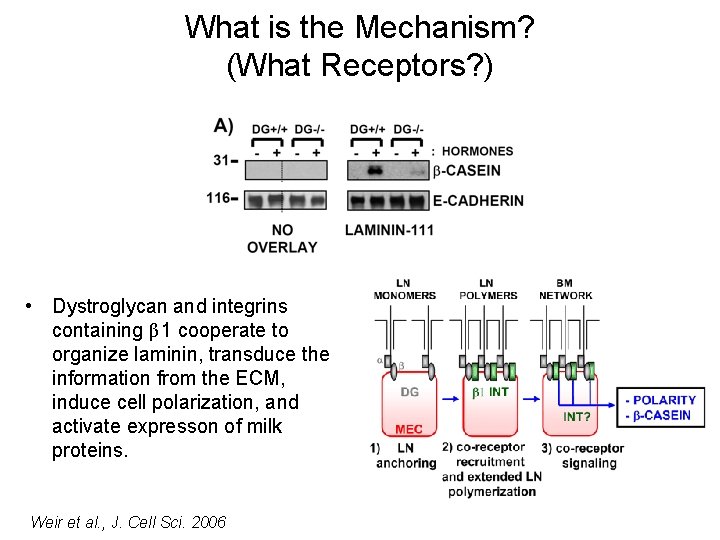
What is the Mechanism? (What Receptors? ) • Dystroglycan and integrins containing β 1 cooperate to organize laminin, transduce the information from the ECM, induce cell polarization, and activate expresson of milk proteins. Weir et al. , J. Cell Sci. 2006