Cell Therapy for Spinal Cord Injuries Commercial Manufacturing
- Slides: 1

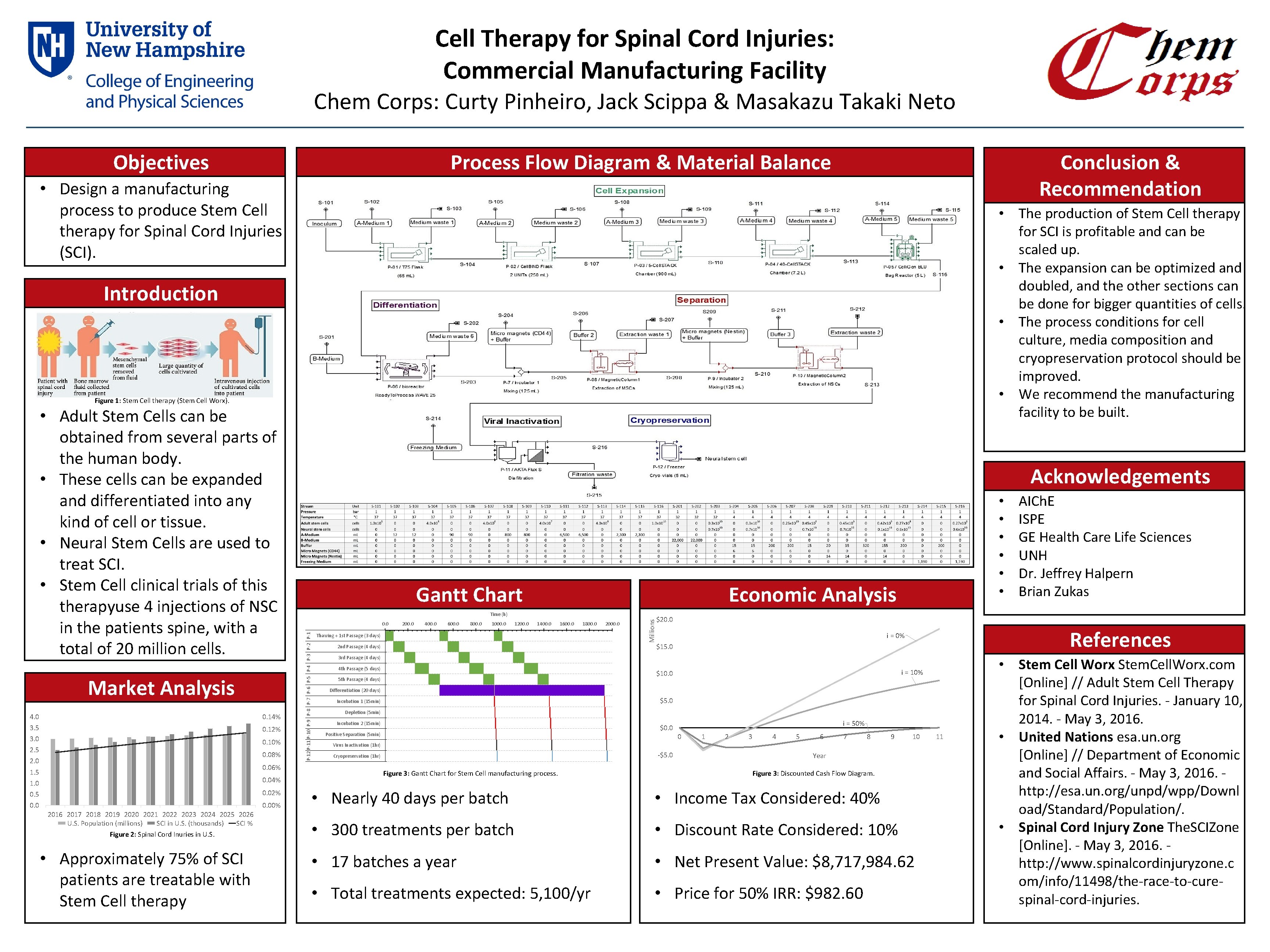
Cell Therapy for Spinal Cord Injuries: Commercial Manufacturing Facility Chem Corps: Curty Pinheiro, Jack Scippa & Masakazu Takaki Neto Conclusion & Recommendation Process Flow Diagram & Material Balance Objectives • Design a manufacturing process to produce Stem Cell therapy for Spinal Cord Injuries (SCI). • The production of Stem Cell therapy for SCI is profitable and can be scaled up. • The expansion can be optimized and doubled, and the other sections can be done for bigger quantities of cells. • The process conditions for cell culture, media composition and cryopreservation protocol should be improved. • We recommend the manufacturing facility to be built. Introduction Figure 1: Stem Cell therapy (Stem Cell Worx). Market Analysis 4. 0 0. 14% 3. 5 0. 12% 3. 0 0. 10% 2. 5 0. 08% 2. 0 Acknowledgements Gantt Chart 0. 06% 1. 5 1. 0 0. 04% 0. 5 0. 02% 0. 00% 2016 2017 2018 2019 2020 2021 2022 2023 2024 2025 2026 U. S. Population (millions) SCI in U. S. (thousands) SCI % Figure 2: Spinal Cord Inuries in U. S. • Approximately 75% of SCI patients are treatable with Stem Cell therapy a 200. 0 400. 0 600. 0 800. 0 1000. 0 1200. 0 1400. 0 1600. 0 1800. 0 Thawing + 1 st Passage (3 days) 2 nd Passage (4 days) 2000. 0 Millions 0. 0 • • • Economic Analysis Time (h) P-12 P-11 P-10 P-9 P-8 P-7 P-6 P-5 P-4 P-3 P-2 P-1 • Adult Stem Cells can be obtained from several parts of the human body. • These cells can be expanded and differentiated into any kind of cell or tissue. • Neural Stem Cells are used to treat SCI. • Stem Cell clinical trials of this therapyuse 4 injections of NSC in the patients spine, with a total of 20 million cells. $20. 0 References i = 0% $15. 0 3 rd Passage (4 days) 4 th Passage (5 days) i = 10% $10. 0 5 th Passage (4 days) Differentiation (20 days) $5. 0 Incubation 1 (15 min) Depletion (5 min) Incubation 2 (15 min) i = 50% $0. 0 Positive Separation (5 min) 0 1 2 3 4 5 6 7 8 9 10 Virus Inactivation (1 hr) -$5. 0 Cryopreservation (1 hr) AICh. E ISPE GE Health Care Life Sciences UNH Dr. Jeffrey Halpern Brian Zukas Figure 3: Gantt Chart for Stem Cell manufacturing process. Year Figure 3: Discounted Cash Flow Diagram. • Nearly 40 days per batch • Income Tax Considered: 40% • 300 treatments per batch • Discount Rate Considered: 10% • 17 batches a year • Net Present Value: $8, 717, 984. 62 • Total treatments expected: 5, 100/yr • Price for 50% IRR: $982. 60 a a 11 • Stem Cell Worx Stem. Cell. Worx. com [Online] // Adult Stem Cell Therapy for Spinal Cord Injuries. - January 10, 2014. - May 3, 2016. • United Nations esa. un. org [Online] // Department of Economic and Social Affairs. - May 3, 2016. - http: //esa. un. org/unpd/wpp/Downl oad/Standard/Population/. • Spinal Cord Injury Zone The. SCIZone [Online]. - May 3, 2016. - http: //www. spinalcordinjuryzone. c om/info/11498/the-race-to-curespinal-cord-injuries.

