Cell suspension culture Cell suspension culture When callus


































- Slides: 51

Cell suspension culture

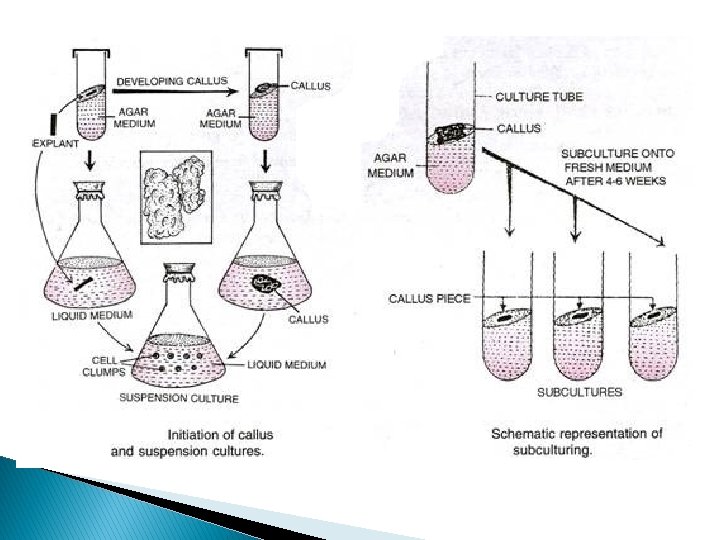
Cell suspension culture • When callus pieces are agitated in a liquid medium, they tend to break up. • Suspensions are much easier to bulk up than callus since there is no manual transfer or solid support. • Large scale (50, 000 l) commercial fermentations for Shikonin and Berberine.



� Aggregates of cells suspended in a liquid medium. � Transfer of callus to liquid media. � Then they are agitated.


FRIABILITY � Separation of cells following cell division. � Good suspension : � Culture consisting of a high percentage of single cells and small clusters of cells. � Different requirements for different cases. � The choice of Suitable conditions is largely determined by trial and error.

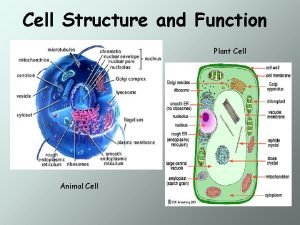
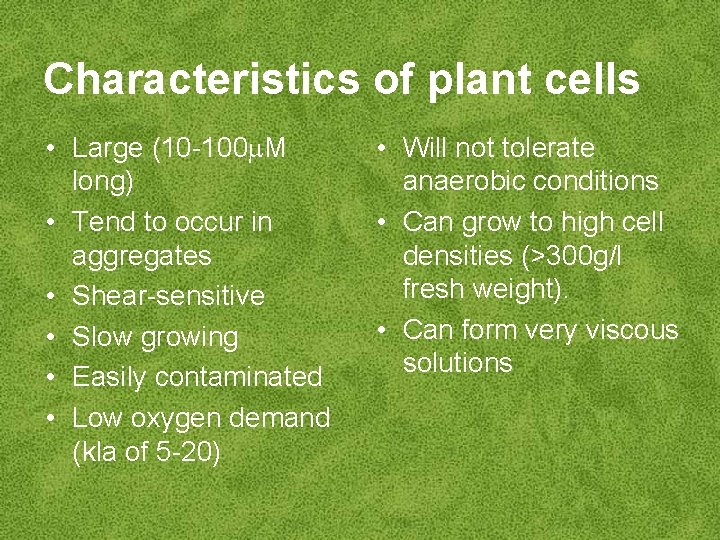
Characteristics of plant cells • Large (10 -100 M long) • Tend to occur in aggregates • Shear-sensitive • Slow growing • Easily contaminated • Low oxygen demand (kla of 5 -20) • Will not tolerate anaerobic conditions • Can grow to high cell densities (>300 g/l fresh weight). • Can form very viscous solutions

� Large amount of callus is required. � 2 -3 gm for 100 cm 3 � Three phases will be observed � Lag phase � Cell division � Stationary phase � The cells should be subcultured early during the stationary phase.

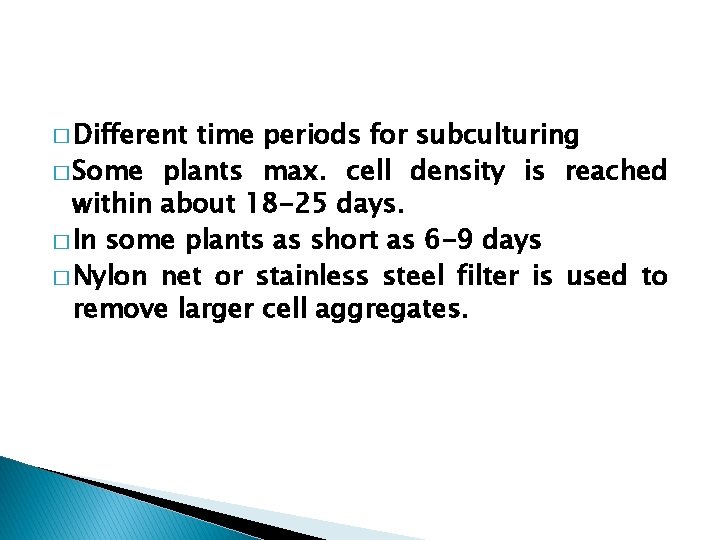
� Different time periods for subculturing � Some plants max. cell density is reached within about 18 -25 days. � In some plants as short as 6 -9 days � Nylon net or stainless steel filter is used to remove larger cell aggregates.

� Small portion is withdrawn and cell density will be checked � 9 -15× 103 cells / cm 3 for sycamore. � The best speed for 100 -120 rpm � Liquid should be filled 20% of the size of the flask for adequate aeration.

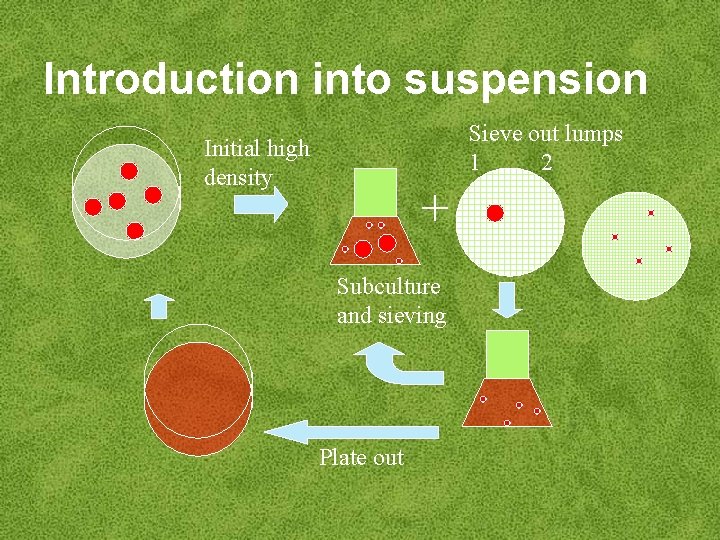
Introduction of callus into suspension • ‘Friable’ callus goes easily into suspension – – 2, 4 -D low cytokinin semi-solid medium enzymic digestion with pectinase – blending • Removal of large cell aggregates by sieving • Plating of single cells and small cell aggregates - only viable cells will grow and can be reintroduced into suspension

Introduction into suspension Sieve out lumps 1 2 Initial high density + Subculture and sieving Plate out

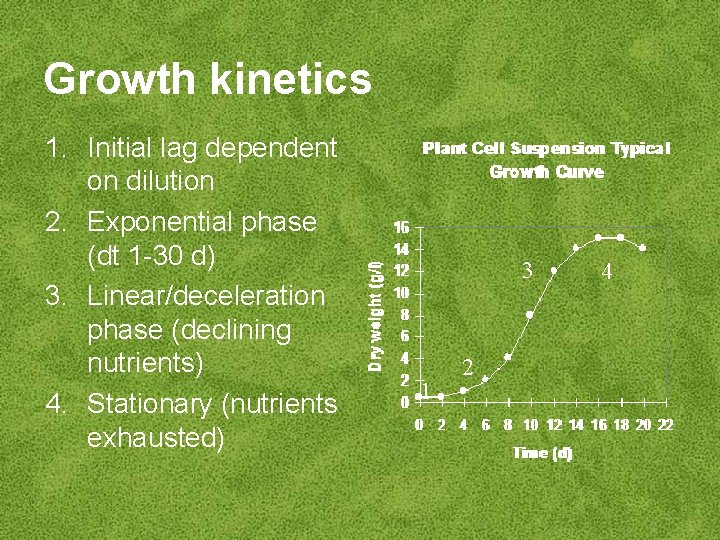
Growth kinetics 1. Initial lag dependent on dilution 2. Exponential phase (dt 1 -30 d) 3. Linear/deceleration phase (declining nutrients) 4. Stationary (nutrients exhausted) 3 1 2 4

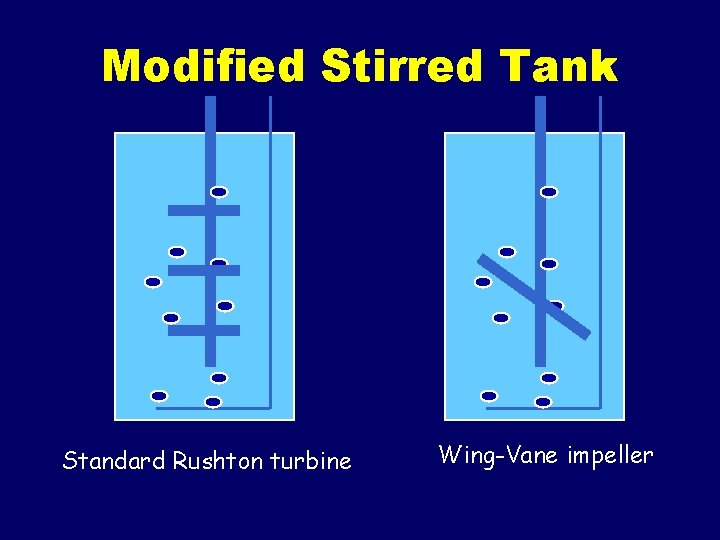
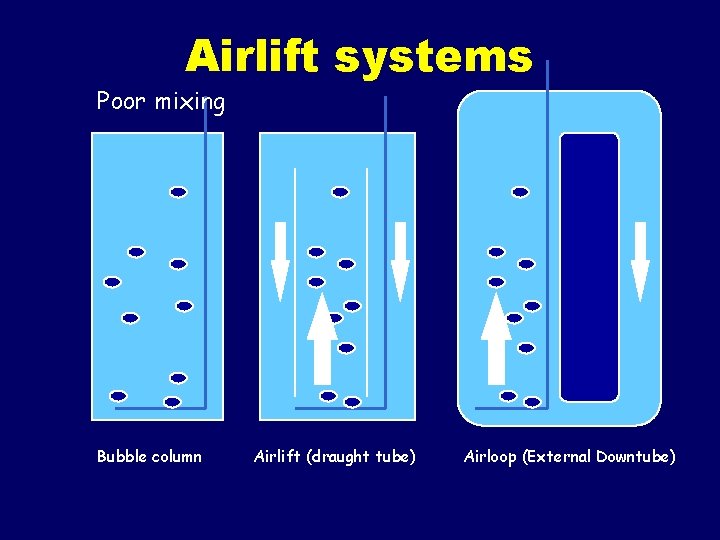
Reactors for plant suspension cultures • • • Modified stirred tank Air-lift Air loop Bubble column Rotating drum reactor

Modified Stirred Tank Standard Rushton turbine Wing-Vane impeller

Airlift systems Poor mixing Bubble column Airlift (draught tube) Airloop (External Downtube)

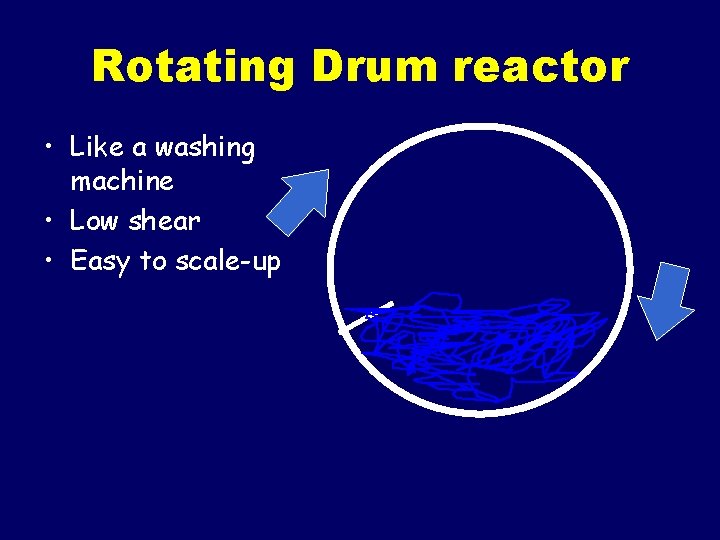
Rotating Drum reactor • Like a washing machine • Low shear • Easy to scale-up

Ways to increase product formation • Select • Start off with a producing part • Modify media for growth and product formation. • Feed precursors or feed intermediates (bioconversion) • Produce ‘plant-like’ conditions (immobilisation)

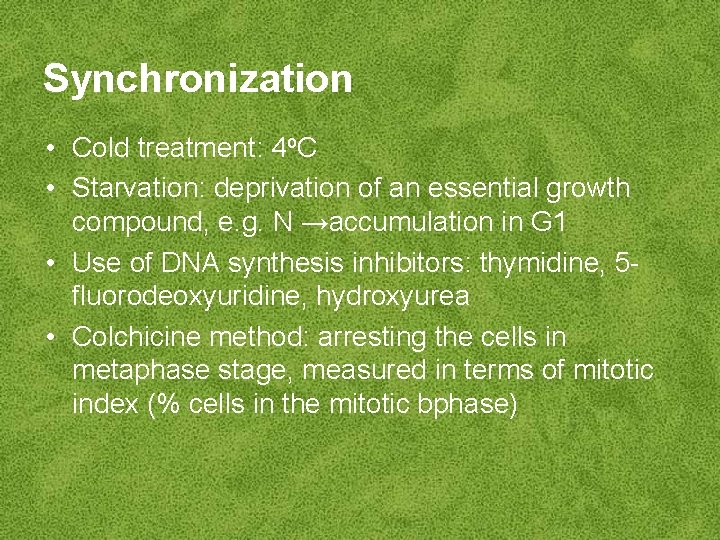
Synchronization • Cold treatment: 4 o. C • Starvation: deprivation of an essential growth compound, e. g. N →accumulation in G 1 • Use of DNA synthesis inhibitors: thymidine, 5 fluorodeoxyuridine, hydroxyurea • Colchicine method: arresting the cells in metaphase stage, measured in terms of mitotic index (% cells in the mitotic bphase)

Selection • Select at the level of the intact plant • Select in culture – single cell is selection unit – possible to plate up to 1, 000 cells on a Petri-dish. – Progressive selection over a number of phases

Selection Strategies • • Positive Negative Visual Analytical Screening

Positive selection • Add into medium a toxic compound e. g. hydroxy proline, kanamycin • Only those cells able to grow in the presence of the selective agent give colonies • Plate out and pick off growing colonies. • Possible to select one colony from millions of plated cells in a days work. • Need a strong selection pressure - get escapes

Negative selection • Add in an agent that kills dividing cells e. g. chlorate / BUd. R. • Plate out leave for a suitable time, wash out agent then put on growth medium. • All cells growing on selective agent will die leaving only non-growing cells to now grow. • Useful for selecting auxotrophs.

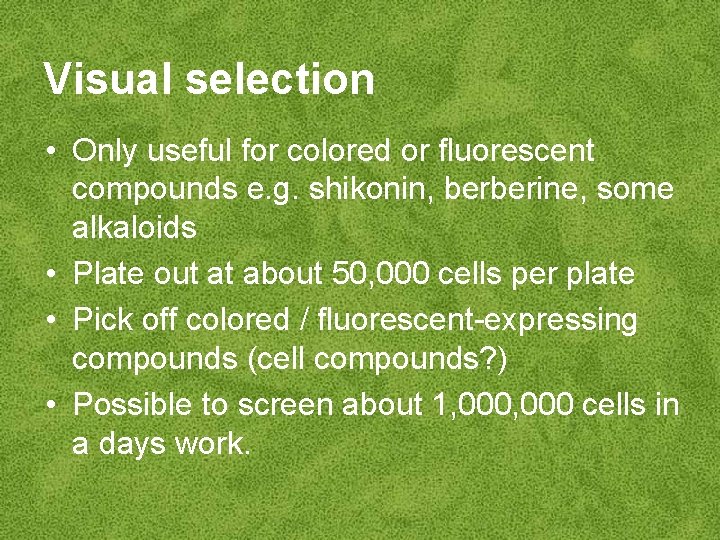
Visual selection • Only useful for colored or fluorescent compounds e. g. shikonin, berberine, some alkaloids • Plate out at about 50, 000 cells per plate • Pick off colored / fluorescent-expressing compounds (cell compounds? ) • Possible to screen about 1, 000 cells in a days work.

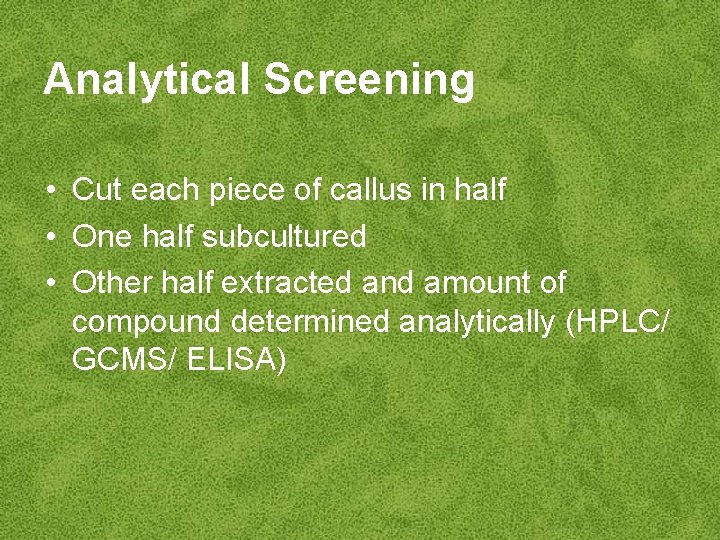
Analytical Screening • Cut each piece of callus in half • One half subcultured • Other half extracted and amount of compound determined analytically (HPLC/ GCMS/ ELISA)

Embryo Culture

Embryo Culture Uses • Rescue F 1 hybrids from wide crosses • Overcome seed dormancy, usually with addition of hormone (GA) to medium • To overcome immaturity in seeds – To speed generations in a breeding program – To rescue a cross or self (valuable genotype)

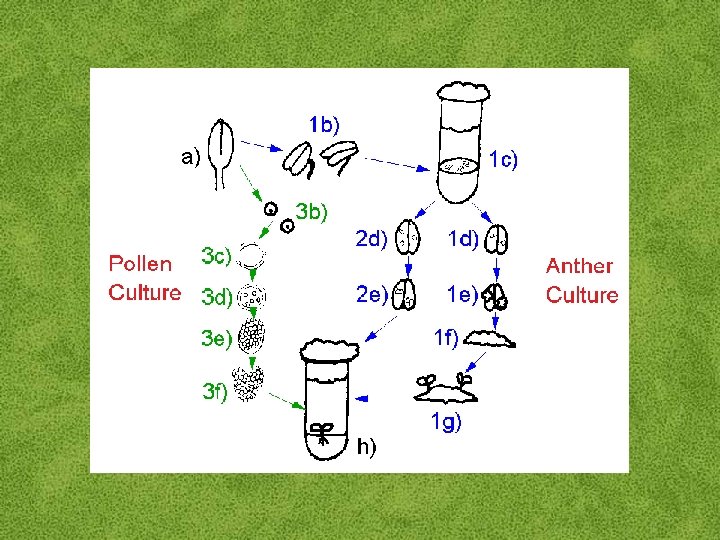
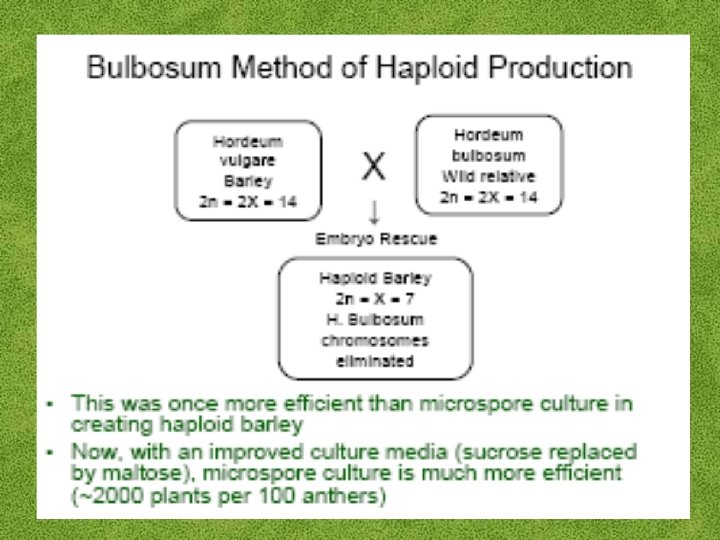
Haploid Plant Production • Embryo rescue of interspecific crosses – Bulbosum method • Anther culture/Microspore culture – Culturing of anthers or pollen grains (microspores) – Derive a mature plant from a single microspore • Ovule culture – Culturing of unfertilized ovules (macrospores)





Anther/Microspore Culture Factors • Genotype • Optimum growth of mother plant • Correct stage of pollen development – Need to be able to switch pollen development from gametogenesis to embryogenesis • Pretreatment of anthers – Cold and heat have been effective • Culture medium – Additives – Agar vs. ‘Floating’

Ovule Culture for Haploid Production • Essentially the same as embryo culture – difference is an unfertilized ovule instead of a fertilized embryo • Effective for crops that do not yet have an efficient microspore culture system – e. g. : melon, onion

Haploids • Weak, sterile plant • Usually want to double the chromosomes, creating a dihaploidbplant with normal growth & fertility – Chromosomes can be doubled by – Colchicine treatment – Spontaneous doubling

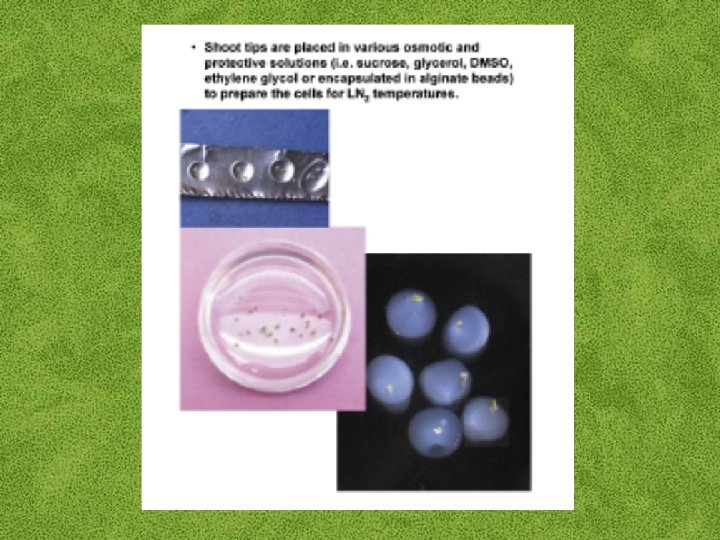
Germplasm Preservation Extension of micropropagation techniques: Two methods: • 1. Slow growth techniques – ↓Temp. , ↓Light, media supplements (osmotic inhibitors, growth retardants), tissue dehydration, etc – Medium-term storage (1 to 4 years) • 2. Cryopreservation – Ultra low temperatures. Stops cell division & metabolic processes – Very long-term (indefinite? )

Most economical germplasm storage – Why not seeds? • Some crops do not produce viable seeds • Some seeds remain viable for a limited duration only and are recalcitrant to storage • Seeds of certain species deteriorate rapidly due to seed borne pathogen • Some seeds are very heterozygous not suitable for maintaining true to type genotypes • Effective approach to circumvent the above problems may be application of cryopreservation technology

Cryogenic explants: • • Undifferentiated plant cells Embryonic suspension Callus Pollen Seeds Somatic embryos Shoot apices

Preparation • • Pretreatment Cryopreservation method Thawing method Recovery method is critical







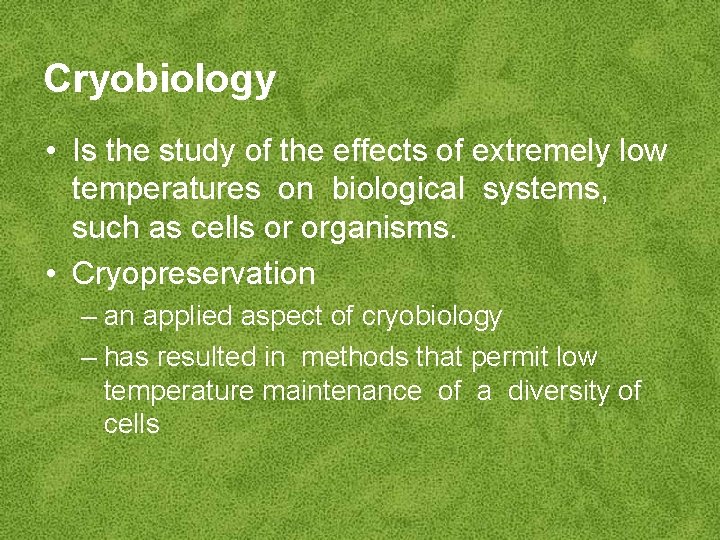
Cryobiology • Is the study of the effects of extremely low temperatures on biological systems, such as cells or organisms. • Cryopreservation – an applied aspect of cryobiology – has resulted in methods that permit low temperature maintenance of a diversity of cells

Cryopreservation Requirements: • Preculturing–Usually a rapid growth rate to create cells with small vacuoles and low water content • Cryoprotection–Glycerol, DMSO, PEG, etc…, to protect against ice damage and alter the form of ice crystals • Freezing–The most critical phase; one of two methods: • Slow freezing allows for cytoplasmic dehydration • Quick freezing results in fast intercellular freezing with little dehydration

Cryopreservation Requirements • Storage–Usually in liquid nitrogen (-96 C) to avoid changes in ice crystals that occur above 100 C • Thawing–Usually rapid thawing to avoid damage from ice crystal growth • Recovery – – Thawed cells must be washed of cryoprotectants and nursed back to normal growth– – Callus production avoided to maintain genetic stability


 Uses of plant tissue culture
Uses of plant tissue culture Plant tissue culture images
Plant tissue culture images Initiation and maintenance of callus culture
Initiation and maintenance of callus culture Applications of plant tissue culture in pharmacognosy
Applications of plant tissue culture in pharmacognosy Callus is
Callus is Antituberkulotikumok
Antituberkulotikumok Callus
Callus Callus
Callus Mr lee van rensburg
Mr lee van rensburg Creeping substitution
Creeping substitution Callus formation
Callus formation Intenzifikált inzulinkezelés
Intenzifikált inzulinkezelés Fibrocartilaginous callus formation
Fibrocartilaginous callus formation Suspension therapy for upper limb
Suspension therapy for upper limb Slowly rotating culture
Slowly rotating culture Material culture example
Material culture example Sociologists define a symbol as
Sociologists define a symbol as Batch culture vs continuous culture
Batch culture vs continuous culture Fed-batch
Fed-batch Individualistic culture definition
Individualistic culture definition American culture vs indian culture
American culture vs indian culture Stab culture and stroke culture
Stab culture and stroke culture Folk culture and popular culture venn diagram
Folk culture and popular culture venn diagram What is a subculture
What is a subculture What is folk culture
What is folk culture Anaerobic medium
Anaerobic medium Folk culture and popular culture venn diagram
Folk culture and popular culture venn diagram In an inert organizational culture,
In an inert organizational culture, Anaerobic gaspak
Anaerobic gaspak Describe lawn culture and surface plating
Describe lawn culture and surface plating Quality culture changing hearts minds and attitudes
Quality culture changing hearts minds and attitudes Surface culture deep culture and esol
Surface culture deep culture and esol Bioreactor considerations for animal cell culture
Bioreactor considerations for animal cell culture Types of animal cell culture
Types of animal cell culture Primary cell culture
Primary cell culture Procedure for isolation of cell for in vitro culture
Procedure for isolation of cell for in vitro culture Cell culture
Cell culture Cell culture
Cell culture Stem cell culture
Stem cell culture Sterile technique cell culture
Sterile technique cell culture Volvox diagram
Volvox diagram Difference between mercury cell and diaphragm cell
Difference between mercury cell and diaphragm cell Site:slidetodoc.com
Site:slidetodoc.com Prokaryotic
Prokaryotic Plant cell animal cell venn diagram
Plant cell animal cell venn diagram What is a half reaction
What is a half reaction Dry cell vs wet cell
Dry cell vs wet cell Venn diagram animal and plant cells
Venn diagram animal and plant cells What is the function of cell wall in plant cell
What is the function of cell wall in plant cell Plant cell structure
Plant cell structure Is cytoplasm in plant and animal cells
Is cytoplasm in plant and animal cells Carbohydrate in cell membrane
Carbohydrate in cell membrane